

Choice of fungicide seed treatment solution composition affecting dust emission from cereal crop seeds
Einfluss der Zusammensetzung der Beizlösung auf die Staubentwicklung an Getreidesaatgut
Journal für Kulturpflanzen, 69 (9). S. 303–308, 2017, ISSN 1867-0911, DOI: 10.1399/JfK.2017.09.03, Verlag Eugen Ulmer KG, Stuttgart
Seed dust emission in small grain cereals is a possible loss path for fungicidal seed dressing and furthermore a source of unrecognized release of chemicals towards non target organisms and users. In the present study, an evaluation of dust emission was conducted due to the use of different slurry solution composition (with and without adjuvant), using different application rates and crop seeds. After using a standardized treatment protocol, sample storage was conducted for an appropriate defined time until dust measurement by Heubach-dustmeter was carried out. Different levels of dust were detected according to the use of adhesive adjuvants. An overall reduction of dust level was achieved by the application of adjuvants compared to the single use of seed dressings.
Key words: Heubach-dustmeter, seed dust emission, slurry solution composition, adjuvants
Die Entstehung von Beizstaub stellt eine potentielle Verlustquelle fungizider Beizmittel dar und kann darüber hinaus eine unbewusste Quelle für die Freisetzung von Chemikalien gegenüber Nichtziel-Organismen und Anwendern bilden. In der vorliegenden Studie wurde eine Bewertung der Staubemission durch die Verwendung von verschiedenen Beizlösungszusammensetzungen (mit und ohne Additiv) anhand verschiedener Aufwandmengen und unterschiedlichem Saatgut durchgeführt. Nach der Verwendung eines standardisierten Behandlungsprotokolls wurde die Probenlagerung für eine definierte Zeit durchgeführt, woraufhin anschließend eine Staubmessung mittels Heubach-Dustmeter erfolgte. Je nach Verwendung von Additiven wurden verschiedene Staubemissionen nachgewiesen. Eine Gesamtreduktion des Staubniveaus wurde durch die Anwendung von Additiven im Vergleich zur alleinigen Verwendung von Saatgutbehandlungsmitteln gezeigt.
Stichwörter: Beizlösungszusammensetzung, Beizstaub, Heubach-Dustmeter, Additiv
Using seed dressings for cereal crop seeds more than 70% of the applications are based on the use of fungicidal agents (Knowles, 2008). The use of sticker-based adhesives in seed treatment differs widely depending on the field of use of liquid components. Robinson and Mayberry already compared different application systems in 1976. Therefore, the treatment of cereal crop seeds is defined to be the simplest form of application because the substances to be applied, are coated on the seed grains in one process, generally without stickers. Other more complex liquid treatments of seeds, such as film coating or pelleting, include more substances that are applied in different layers and need conventional adhesive stickers or binder (Halmer, 1988). Contrary to this, the use of adhesives for cereal seed treatment is not a need of procedure, but rather an improvement of biological effectiveness, which was already mentioned in field applications (Foy, 1989; Hazen, 2000). Thus the products, being applied as a supplement in cereal seed treatment can also be declared as “adjuvants” (Tann, 2010; Hochberg, 1996).
An uncontrolled leakage of plant protection product always forms a potential source of contamination to the environment and can furthermore be an unnoticed intoxication for user. The development of dust from seed kernels can be such a source, when being treated with agrochemicals (Nuyttens et al., 2013). Although the treatment of seeds with chemical agents, preventing plant losses by pests and diseases, is considered to be a precise way to apply plant protection products (Tjamos et al., 1991), the application of neonicotinoid insecticides negatively affected organisms and caused worldwide mortality of honey bees in recent years (Pistorius et al., 2010). Depending on the indication, the amount of active ingredients per single seed kernel is higher for insecticidal substances, compared to those of fungicidal use. Therefore, the contribution of different stickers towards adhesive capacity, to bind chemical and biological agents to kernel surface, was already mentioned (Scott, 1989; Taylor and Harman, 1990).
In this study the seed dust emission from fungicidal seed dressings in different cereal crop seeds was examined. Therefore an investigation concerning a laboratory seed treatment trial with uniform determined application rates for all cereals was conducted. Additionally the contribution of seed dressing adhesive adjuvants was analysed towards it's technical potential to change adhesive strength of seed treatment slurry and compared to single use of seed dressings.
The trial was carried out using commercial small grain cereal untreated seeds. Certified seeds with a legally minimal required technical purity of 98% from winter wheat (cultivar: Potenzial, Breeder: Deutsche Saatgutveredelung AG, Lippstadt GER), winter barley (cultivar: KWS Kosmos, Breeder: KWS Lochow GmbH, Bergen GER), winter rye (cultivar: Brasetto, Breeder: KWS Lochow GmbH, Bergen GER), oats (cultivar: Bison, Breeder: Saaten-Union GmbH, Isernhagen GER) and winter triticale (cultivar: Cosinus, Breeder: KWS Lochow GmbH, Bergen GER) were used.
Seed dressings, chosen for the trial, were universal suspension concentrates (SC) EfA® (Fluoxastrobin 37,5 g l–1, Prothioconazole 25,0 g l–1, Triazoxide 10,0 g l–1, Tebuconazole 3,75 g l–1; company: BayerCrop Science, Mohnheim, GER) and Rubin®TT (Pyrimethanile 42,0 g l–1, Triticonazol 25,0 g l–1, Prochloraz 38,6 g l–1; company: BASF Crop Protection, Limburgerhof, GER), authorized for use in all crops taken for the trial. Additionally, three seed treatment adjuvants were used in the trial, two emulsifiable concentrates (EC), Inteco® (company: BayerCrop Science, Mohnheim, GER) and Kantor® (company: Agroplanta GmbH & Co. KG, Langenpreising-Zustorf, GER), and one suspension concentrate (SC), MaximalFlow® (company: BASF Crop Protection, Limburgerhof, GER). Inteco® mainly consists of soybean oil, while Kantor® is mainly composed of alkoxylated triglycerides. The major ingredient of MaximalFlow® is a polymer dispersion based on silicone oil.
The treatment solution was mixed in a 15 ml centrifuge tube with screw cap. First the seed dressing was added with variable rates ranging from 100 μl per 100 g to 300 μl per 100 g with a stepwise increase of 50 μl (100 μl per 100 g, 150 μl per 100 g, 200 μl per 100 g, 250 μl per 100 g, 300 μl per 100 g). A constant input of 40 μl per 100 g was contributed to slurry production when adding adjuvants. At least the difference from individual ingredient based slurry amount was filled up to a defined level with tap water to get a fix amount for seed application in each variant.
Specific amount of seed dressing |
(100, 150, 200, 250, 300 μl per 100 g) |
|
+ |
partial addition of adjuvants |
(40 μl per 100 g) |
+ |
rest amount of tap water |
(900, 850, 800, 750, 700 μl per 100 g) |
(860, 810, 760, 710, 660 μl per 100 g) |
||
= |
1000 μl per 100 g slurry for seed treatment |
|
Seed treatment process was carried out using a laboratory batch seed treater Hege 11 (Wintersteiger). The treatment process was standardized for application time and volume as for mixing time. Pre application time was about 4 s, followed by a 12 s application time and ending with a mixing time of 4 s. The batch to be treated was 400 g for each variant. Application was uniformly executed using a 5 ml syringes (Braun) with a constant application rate of 1 ml treatment solution per 100 g seeds.
Seed dust measurements were carried out using a Heubach-dustmeter (Type I). This engine simulates the mechanical stress of seeds after treatment process caused by transport or sowing. Methodology was used from specification of the Julius Kühn-Institut (Heimbach, 2011). After treatment process the seeds were stored in 1.5 kg paper bags for two days at 20 ± 2°C with a relative humidity of 50 ± 10%. Instrument settings were standardized for revolutions per minute (30), air flow per minute (20 l) and for revolution period (120 sec). A GF 92 Whatman glass fibre filter with inorganic binder was used to capture developing dust emission. An amount of 100 g of seeds was inserted into the abrasion drum. To evaluate the development of dust, a differential weighting for the whole filter holder, including the filter, was conducted before and after abrasion test. For each variant three replicates were performed. The average forms the Heubach value.
The statistical software R (2015) was used to evaluate the data. The data evaluation started with the definition of an appropriate linear model. The data were assumed to be normally distributed and to be homoscedastic. These assumptions are based on a graphical residual analysis. First, a statistical model with the factors crop seed, seed dressing and adjuvant, and covariate application rate, including all possible interaction terms (two-fold and three-fold), was used. Based on this model, an analysis of variance (ANOVA) was conducted, followed by multiple contrast tests (Bretz et al., 2010) in order to compare the several compositions of seed dressings at the same application rate.
An ANOVA was conducted evaluating the significance of the different single factors and each interaction. The analysis of variance provides significant evidence to several factors and interactions (Table 1). The two fold interactions seed dressing and application rate (p = 0.019460) also as adjuvant and application rate (p = 2.688e–13) were not significant.
Table 1. Analysis of variance (ANOVA) for several factors and interactions
Df | sum sq | mean sq | F value | Pr(>F) | ||
crop seed | 4 | 12.281 | 3.0702 | 452.1109 | < 2.2e–16 | *** |
seed dressing | 1 | 0.053 | 0.0530 | 7.8078 | 0.005452 | ** |
adjuvant | 3 | 64.295 | 21.4316 | 3155.9759 | < 2.2e–16 | *** |
application rate | 4 | 0.119 | 0.0298 | 4.3851 | 0.001760 | ** |
crop seed:seed dressing | 4 | 1.025 | 0.2562 | 37.7233 | < 2.2e–16 | *** |
crop seed:adjuvant | 12 | 9.049 | 0.7541 | 111.0488 | < 2.2e–16 | *** |
seed dressing:adjuvant | 3 | 0.563 | 0.1877 | 27.6389 | 2.910e–16 | *** |
crop seed:application rate | 16 | 1.728 | 0.1080 | 15.9080 | < 2.2e–16 | *** |
seed dressing:application rate | 4 | 0.081 | 0.0202 | 2.9689 | 0.019460 | |
adjuvant:application rate | 12 | 0.644 | 0.0537 | 7.9073 | 2.688e–13 | |
crop seed:seed dressing:adjuvant | 12 | 0.941 | 0.0784 | 11.5414 | < 2.2e–16 | *** |
crop seed:seed dressing:application rate | 16 | 0.204 | 0.0128 | 1.8791 | 0.020825 | * |
crop seed:adjuvant:application rate | 48 | 1.653 | 0.0344 | 5.0716 | < 2.2e–16 | *** |
seed dressing:adjuvant:application rate | 12 | 0.354 | 0.0295 | 4.3460 | 1.625e–06 | *** |
crop seed:seed dressing:adjuvant: application rate | 48 | 1.533 | 0.0319 | 4.7017 | < 2.2e–16 | *** |
residuals | 400 | 2.716 | 0.0068 | |||
Signif. codes: ≤ 0.001 ***; ≤ 0.01 **; ≤ 0.05 * | ||||||
The use of different seed dressings (EfA®, Rubin®TT) in five different cultures resulted in different levels of abrasion. The columns in Figure 1 and Figure 2 show the comparison between different solution compositions depending on the addition of adjuvants for each application rate. The use of all adjuvants exhibited an overall reduction of dust emission compared to the single use of the seed dressing. Statistical significant differences were found for all combinations besides Rubin®TT and Kantor® for the lowest application rate (100 μl per 100 g), applied in wheat seeds and for the combination Rubin®TT and MaximalFlow® at the highest application rate (300 μl per 100 g), applied in oat.
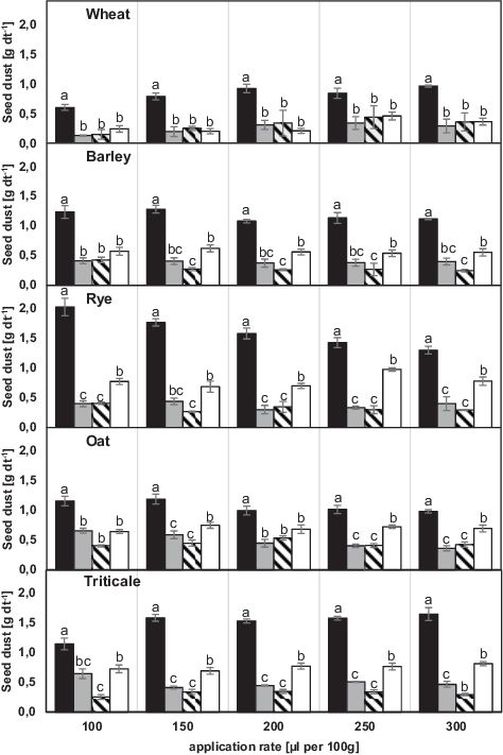
Fig. 1. Seed dust from EfA® treated seed using the Heubach-test in four seed dressing compositions (single, +Kantor®, +Inteco®, +MaximalFlow®; black, grey, striped, white) depending on application rate (100 μl per 100 g, 150 μl per 100 g, 200 μl per 100 g, 250 μl per 100 g, 300 μl per 100 g) and crop seeds (wheat, barley, rye, oat, triticale).
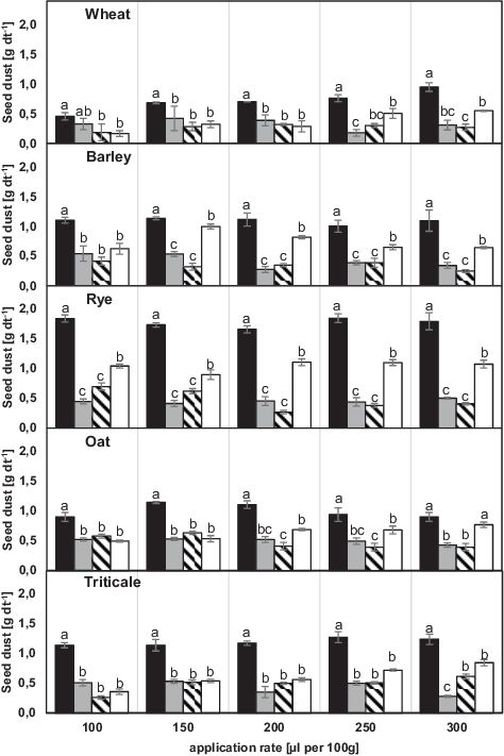
Fig. 2. Seed dust from Rubin®TT treated seed using the Heubach-test in four seed dressing compositions (single, +Kantor®, +Inteco®, +MaximalFlow®; black, grey, striped, white) depending on application rate (100 μl per 100 g, 150 μl per 100 g, 200 μl per 100 g, 250 μl per 100 g, 300 μl per 100 g) and crop seeds (wheat, barley, rye, oat, triticale).
Differences between crops are visible regarding the single use of the seed dressings EfA® and Rubin®TT. The lowest amount of abrasion that was detected for all crops was measured using the seed dressing Rubin®TT with an application rate of 100 μl per 100 g in wheat, the highest for EfA® with an application rate of 100 μl per 100 g in rye.
Statistical significant differences between the adjuvant added variants could be found in several fourfold interaction of seed dressing, adjuvant, crop seed and application rate.
No specific interaction of seed dressing and adhesive adjuvant was found in wheat using EfA®. For seed dressing Rubin®TT significant differences concerning the applied adjuvant were found at highest application rates 250 μl per 100 g and 300 μl per 100 g. Regardless of seed dressing used for crop seeds of barley no significance was detected between single adjuvants at lowest application rate. For both seed dressings there was a significant difference between MaximalFlow® and Inteco® for the application rates 150 μl per 100 g up to 300 μl per 100 g. A significant difference between the adjuvants Kantor® and MaximalFlow® was only determined for Rubin®TT. For both seed dressings applied in rye, the combination with Kantor® and Inteco® resulted in lower dust emission levels than using MaximalFlow®. Besides the combination EfA® and Kantor® at the application rate 150 μl per 100 g there was an overall significant difference between MaximalFlow® and Kantor® or Inteco®. At lowest application level no specific interaction between seed dressing and adjuvant was determined for use in oat. Significant differences between adjuvants were detected at the highest application level for both seed dressings and at 250 μl per 100 g for EfA®. Therefore the combination with the adjuvant MaximalFlow® indicated statistical significant higher amounts of dust, compared to at least one of the alternative adjuvants. The use of the different adjuvants in triticale resulted in lower abrasion levels for seed dressing EfA®. Besides application level of 100 μl per 100 g a significant difference between MaximalFlow® and Inteco® or Kantor® was observed. For seed dressing Rubin®TT the only significant difference between the adjuvant added variants was detected at highest application rate.
The Heubach abrasion test is the standard process to estimate potential losses of seed dressings through fine dust. Nevertheless this method does not capture the whole dust losses. Especially for cultures with glumes (rye and oat) there is a second loss path in form of coarse dust, which was already mentioned for insecticides in maize (Pistorius et al., 2010). However, the risk of user inhalation is higher for fine dust (Heinrich et al., 2002). It is to be assumed, that parts of the fine dust are respirable particulates, which can (according to DIN ISO 7708) penetrate the air tube to the lungs and cross the alveoli into the bloodstream, so that there is a potential danger to the user, besides environmental risks. Thus a strategy of avoidance is necessary.
Depending on the addition of the various adhesive adjuvants, different amounts of abrasion could be measured as a result of surface modification, which differed in relation to the added adhesives. The use of the universal suspension concentrates EfA® and Rubin®TT in combination with the adhesive adjuvants Kantor®, MaximalFlow® and Inteco® resulted in a significant reduction of dust emission compared to the single use of seed dressing in all crop seeds at the most application rates. This is common to studies from Schnier et al. (2003). The use of adjuvants led to lower abrasion of chemically treated maize seeds with a defined application rate. Complementary, because the reducing dust emissions have been detected in all cereal crop seeds that were used in the present study, it is to be assumed that the improved adhesion of chemicals due to the addition of adhesive adjuvants is expected to be irrespective of the genetic conditions of the grains such as grain shape and surface texture.
Regarding the current approach from farmers position, the use of adhesive adjuvants could be a reliable way to reach the target quantity for a defined indication against pests and diseases, due to lower losses. However, if the differences have an effect on the indication is not known yet for cereal products. Against that the impact of adjuvants to bind organic matter to seeds was already mentioned for sugar beet, resulting in an improved protection against soil borne diseases (Bardin and Huang, 2003). Contrary to cereal seed treatment the use of adjuvants for sugar beet pelleting is necessary and not only a technical benefit. The improved adhesion of all used seed dressings due to the use of adjuvants in the present study might also lead to a lower risk to harm non target organisms. This would mean an improvement of the seed treatment quality or abrasion resistance. Nikolakis et al. (2009) confirmed the seed treatment quality to be an important influencing factor to cause dust drift in fields after sowing. The fact that there is an optimization potential for existing products and application systems at different application rates can help to increase the safety of the already secure and efficient method of seed treatment, compared to field applications (Taylor and Harman, 1990; Matyjaszczyk and Piecynska, 2015).
The amounts of dust emissions at a defined application rate can thus be influenced by modifications of the slurry solution composition and represents an additional possibility to generate an optimization of the adhesion, that directly interacts into the treatment process. In addition to this change, dust-reducing effects have already been detected using technical transformations. In 2011 Biocca et al. evaluated an alternative exhaust air diverter for pneumatic sowing machines. The deflection near the ground led to a lower release of active substances into the environment (Pochi et al., 2012). A less weather-independent conversion was developed in 2015, using a filter system on existing sowing machines as a dust-catching optimization (Biocca et al., 2015). In contrast, a change of the slurry solution composition could allow dust reduction in all given sowing techniques. Contrary to this, technical transformation also allows the use of higher emitting seed batches. However, both approaches represent a strategy of avoidance.
Differences between dust emitting potential of different crops might be affected by surface texture of the single kernels. Various differences between wax proportion of the bran, the homogenicity of tube cells at the kernel surface or the existence of glumes are given between the cereal crops (Möller and Wintons, 1905), so that there might be individual interactions to the applied slurry and to holding capacity.
The use of different adjuvant resulted in different levels of abrasion. Several comparisons indicate, that the improvement of adhesive strength was lower when using the polysiloxane based adjuvant MaximalFlow®. A low adhesion of silicone oils has already been demonstrated in field applications. Penner et al. (2002) found that there was no increase in retention on the leaves, but a contrary rejection of the spray drops. However, there is no complete transferability to the documented effects in a seed treatment process. Additionally, there is a tendency, that the use of vegetable oil based adjuvants, such as soybean oil (Inteco®, + Kantor), is more suitable to lower dust emissions. The ability of vegetable oils to bind dust and to reduce it´s development is a benefit that is already used in feeding strategies to improve air quality in swine confinement buildings (Heber, 2002; Guarino et al., 2007).
Although the high number of comparisons between the single use and a adjuvant added application of slurry composition indicate a lower dust emission when using adjuvants, a week point of the interpretation might be, that the samples for dust emission analysis in this study result from only one batch, so that each crop variant only contain technical replicates.
The addition of adhesive adjuvants can reduce the amount of dust emission compared to single use of fungicide seed treatment in small grain cereals. The dimension of reduction depends on the used adjuvant. Specific interactions between influential factors cause differences between the additional agents. Further studies are required to validate the interacting effects of seed dressing and crop seeds as the interaction of seed dressing, crop seeds and used adjuvant.
Bardin, S.D., H.C. Huang, 2003: Efficacy of Stickers for Seed Treatment with Organic Matter or Microbial Agents for the Control of Damping-off of Sugar Beet. Plant Pathology Bulletin. 12, 19-26.
Biocca, M., E. Conte, P. Pulcini, E. Marinelli, D. Pochi, 2011: Sowing simulation tests of a pneumatic drill equipped with systems aimed at reducing the emission of abrasion dust from maize dressed seed. J. Environ. Sci. Health 46 (6), 438-448.
Biocca. M., D. Pochi, R. Fanigliulo, P. Gallo, 2015: Dust emissions during the sowing of maize dressed seeds and drift reducing devices. The Open Agriculture Journal 9, 42-47.
Bretz, F., T. Hothorn, P. Westfall, 2010: Multiple Comparisons using R. s.l.: Chapman and Hall/CRC.
Foy, C.L., 1989: Adjuvants for agrochemicals: introduction, historical overview, and future outlook. in: Adjuvants and Agrochemicals, Vol. II, Recent Development, Application and Bibliography of Agro-adjuvants, ed. by P. Chow, C. Grant, A. Hinshalwood, E. Simundsson. Boca Raton FL, CRC Press,,, 9-13.
Friessleben, R., T. Schad, R. Schmuck, H. Schnier, R. Schöning, A. Nikolakis, 2010: An effective riskmanagement approach to prevent bee damage due to the emission of abraded seed treatment particles during sowing of neonicotinoid treated maize seeds. Aspects Appl. Biol. 99, 277-282.
Guarino, M., L.D. Jacobson, K.A. Janni, 2007: Dust Reduction from oil based Feed Adjuvants. Applied Engineering in Agriculture 23 (3), 329-332.
Hazen, J.L., 2000: Adjuvants – terminology, classification and chemistry. Weed Technol. 14, 773-784.
Heber, A.J., 2002: Effects of high-oil corn and soybean oil adjuvants on dustiness of ground corn and feed. Applied Engineering in Agriculture. 45 (5), 1593-1598.
Heimbach, U., 2011: Heubach Method to Determine the Particulate Matter of Maize Seeds Treated with Insecticides. http://www.jki.bund.de/heubachen.html. [Online] Abrufdatum?.
Heinrich, J., V. Grote, A. Peters, E.H. Wichmann, 2002: Gesundheitliche Wirkungen von Feinstaub: Epidemiologie der Langzeiteffekte. Umweltmedizin in Forschung und Praxis 7 (2), 91-99.
Hochberg, E.G., 1996: The market for Agricultural pesticide inert ingredients and adjuvants. In: Pritchard D.W., Foy C.L., Pesticide Formulation and Adjuvant Technology. Boca Raton, NY, CRC Press, pp. 203-209.
Knowles, A., 2008: Recent developments of safer formulations of agrochemicals. Environmentalist, 28 (1): 35-44.
Matyjaszczyk, E., A. Piecynska, 2015: The use of an active substance depending on the application method of plant protection products: seed dressing versus foliar treatment. Agriculture and Agricultural Science Procedia 7,165-169.
Möller, J., A.L. Wintons, 1905: Mikroskopie der Nahrungs- und Genussmittel aus dem Pflanzenreiche. 2. Auflage. Berlin, Heidelberg, Springer Verlag.
Nikolakis, A., A. Chapple, R. Friesleben, P. Neumann, T. Schad, R. Schmuck, H.-F. Schnier, H.-J. Schnorbach, R. Schöning, C. Maus, 2009: An effective risk management approach to prevent bee damage due to the emission of abraded seed treatment particles during sowing of seeds treated with bee toxic insecticides. Julius-Kühn-Archiv 423, 132-148.
Nuyttens, D., W. Devarrewaere, P. Verboven, D. Foque, 2013: Pesticide-laden dust emission and drift from treated seeds during seed drilling: a review. Pest. Manag. Sci. 69, 564-575.
Pochi, D., M. Biocca, R. Fanigliulo, P. Pulcini, E. Conte, 2012: Potential exposure of bees, Aphis mellifera L., to particulate matter and pesticides derived from seed dressing during maize sowing. Bull. Environ. Contam. Toxicol. 89, 354-361.
Pistorius, J., G. Bischoff, U. Heimbach, M. Stähler, 2010: Bee poisoning incidents in Germany in spring 2008 caused by abrasion of active substance from treated seeds during sowing of maize. Julius-Kühn-Archiv 423, 118-126.
Robinson, F.E., K.S. Mayberry, 1976: Seed coating, precision planting and sprinkler irrigation for optimum stand establishment. Agron. J., 68, 694-695.
Scott, J.M., 1989: Seed coatings and treatments and their effectson plant establishment. Adv. Agron. 42, 43-83.
Schnier, H.F., G. Wenig, F. Laubert, V. Simon, R. Schmuck, 2003: Honey bee safety of imidacloprid corn seed treatment. Bulletin of Insectology, 56 (1), 73-75.
Tann, R., 2010: Adjuvant classification – chemistry, functionality, terminology. In: 9th International Symposium on Adjuvants for Agrochemicals. Ed. by P. Baur und M. Bonnet, Freising, Germany. ISAA, 27-36.
Taylor, A.G., G.E. Harman, 1990: Concepts and technologies of selected seed treatments. Annual Review of Phytopathology 28, 321-339.
Tjamos, E.C., G.C. Papavizas, R.J. Cook, 1991: Biological Control of Plant Diseases. New York, Springer Science + Business Media New York.