

Effects of water salinity on germination and early seedling growth of untreated and pelleted sugar beet seeds (Beta vulgaris L.)
Einfluss von Salzstress auf Keimung und Keimpflanzenwachstum von nacktem und unbehandeltem Zuckerrüben-Saatgut (Beta vulgaris L.)
Journal für Kulturpflanzen, 70 (10/11). S. 305–313, 2018, ISSN 1867-0911, DOI: 10.1399/JfK.2018.10-11.01, Verlag Eugen Ulmer KG, Stuttgart
Salinity is a serious abiotic stress factor as it restricts growth of many field crops, especially in the arid and semi-arid areas of the world. In this study, the effects of salinity stress on germination and early seedling growth of sugar beet seeds of different cultivars and breeding lines under laboratory conditions are investigated. Untreated and pelleted seeds of two sugar beet cultivars and five breeding lines were germinated at six salinity levels. The salinity levels were defined by electrical conductivity (EC): distilled water (~0 dS/m), and NaCl solutions of 2, 4, 6, 8, and 10 dS/m. In general, the results indicated that the salinity levels did not affect final germination percentage, but affected significantly emergence percentage, mean germination time and seedling fresh weight compared to control (~0 dS/m). Seedling emergence percentage was significantly reduced at salinity level 10 dS/m. Seedling fresh weight was significantly enhanced by all salinity levels compared to control. Mean germination time was delayed significantly due to increasing salinity levels. Under field growth condition, reduced emergence may affect the ability of the plants to compete for space, water and nutrients with weeds, or prolongs the time of vulnerability to pests and diseases. Therefore, it is recommended that besides the overall salinity resistance, seedling emergence under salt stress should be considered in breeding programs.
Key words: sugar beet, salt stress, germination, emergence
Salinität ist ein abiotischer Einflussfaktor, durch den insbesondere in ariden und semi-ariden Regionen das Wachstum vieler landwirtschaftlicher Kulturen begrenzt wird. Es wurde mit Hilfe von Keimtests untersucht, wie sich unterschiedliche Salzkonzentrationen und Saatgutbeizung auf die Keimung von Zuckerrübensamen und das Wachstum der Keimpflanzen auswirken. Die Sämlinge von zwei Zuckerrübensorten und fünf verschiedenen Zuckerrüben-Genotypen wurden in Keimrollen unter Laborbedingungen angezogen. Es wurden NaCl-Lösungen mit einer elektrischen Leitfähigkeit von 2, 4, 6, 8 und 10 dS/m hergestellt und an Saatgut mit und ohne Pelletierung getestet; als Kontrolle diente destilliertes Wasser (~0 dS/m).
Auf die Keimung der Zuckerrübensamen hatte die Salzkonzentration der Anzuchtlösungen zunächst nur geringen Einfluss. Jedoch wurde die weitere Entwicklung der Keimpflanzen durch höhere Salinität deutlich beeinflusst. Das Keimlingswachstum wurde signifikant gehemmt, das Frischmassegewicht der Sämlinge jedoch gefördert. Unter Feldbedingungen kann sich eine verzögerte Jugendentwicklung negativ auf die Konkurrenzfähigkeit der Kulturpflanzen gegenüber Beikräutern um Raum, Licht, Wasser und Nährstoffe auswirken, oder die Zeiten der Empfänglichkeit für Schädlinge und/oder Krankheiten verlängern. Es erscheint daher lohnend nicht nur die Salztoleranz insgesamt, sondern auch deren Interaktion mit der frühen Jugendentwicklung der Pflanzen in Zuchtprogrammen zu berücksichtigen.
Stichwörter: Zuckerrübe, Salztoleranz, Jugendentwicklung
Especially in arid and semi arid regions, salinity in soil or water is one of the major stress factors that can severely limit crop productivity (Shannon, 1998). Natural or human induced processes are the basic cause for salinity. Dissolved salts will accumulate in the soil water to an extent that inhibits plant growth.
Secondary salt affected soils are those that have been salinized by human caused factors, mainly because of improper irrigation methods. Water of poor quality is often used for irrigation resulting in increasing salt content in the upper soil unless the management of irrigation system is such that salts are leached from the soil profile (Yadav et al., 2011). The impact of salinity varies with plant species, stage of growth, management practices, soil types and soil fertility. Salts affect plants through direct ion toxicity to plant tissues, reduced availability of essential elements, effects on the osmosis, and through inhibition of the soil water extraction by plant roots (Podmore, 2009). Salt tolerance in glycophytes is associated with the ability to limit uptake and/or transport of saline ions (mainly Na+ and Cl from the roots to aerial parts (Greenway and Munns, 1980)). Brady and Weil (2002) grouped plant species into four salinity classes by the EC: sensitive crops can tolerate salt levels between 0–4 dS/m, moderately tolerant crops 4–6 dS/m, tolerant crops 6–8 dS/m, and highly tolerant crops 8–12 dS/m.
Seed pelleting may affect germination, field emergence and the crop establishment of sugar beet (Beta vulgaris L.). Because of its uniformity and high density pelleted sugar beet seeds are advantageous for precision planting. Seed enhancement technologies, i.e. priming, pelleting and coating have been extensively used throughout the last century to improve crop yield and to reduce losses associated with pest infestation (Blunk, et. al., 2017). The degree of singleness can be controlled by seed pelleting too. Fungicides and fertilizers are also incorporated in some types of pellets.
Sugar beet is a crop cultivated for production of sugar and energy (bio-ethanol). It can be grown on a wide range of soils and in different climates and provides about 16% of the world's sugar production. The world production in the year 2016 was about 277 million tons of sugar beets from 4.6 million ha (FAOSTAT, 2018).
Prerequisite for the establishment of highly productive sugar beet crop stands is high and uniform field emergence. Seed germination is the most critical stage, followed by the early development of the seedling up to the complete field emergence. Many studies evaluated the rate of growth and yield of many crops under increasing salinity levels and concluded that salinity retards plant growth and reduces yield (Khayami et. al., 2014, Mosta et. al., 2012, and Al-Yahyai et. al., 2010).
Although sugar beet is one of the most salinity tolerant crops, it is less tolerant during germination and emergence (Kaffka and Hembree, 2004). Under field growth conditions reduced emergence may affect the ability of the plants to compete for space, water and nutrients with weeds (Haneklaus et al., 2018), or prolonge the time of vulnerability to pests and diseases. The aim of this study was to test the effects of water salinity levels on seed germination and emergence of untreated and pelleted seeds of two sugar beet cultivars and five breeding lines.
The germination experiments were carried out in the laboratory at Julius Kühn-Institut, Federal Research Centre for Cultivated Plants, in Braunschweig (Germany) during 15/9/2014–31/10/2014.
The untreated and pelleted seed samples were provided by Strube Research, a seed producer from Söllingen, Germany. Two sugar beet cultivars ‘Ballade’ and ‘Lenard’ already attributed by their breeders for their potential resistance against salinity and five breeding lines ST-SUD-01/14, ST-SUD-04/14, ST-SUD-05/14, ST-SUD-06/14, and ST-SUD-07/14 were tested. All pelleted seeds were treated at Strube Research with a combination of Aatiram 65-Thiram, Tachigaren 70 WP, Hymexazol, and Gaucho 70 WP (Imidacloprid).
The salt solutions for moistening the seed rolls were adjusted to EC levels of 2, 4, 6, 8, and 10 dS/m by using NaCl. The electrical conductivity (EC, dS/m) for each NaCl concentration was measured using a precision conductivity meter (conductivity cell Tetra Con 96; WTW). NaCl concentrations were plotted against measured EC and a regression analysis was performed. The following equation was used for the calculation:
Distilled water with an EC (~0 dS/m) served as the control treatment.
The treatments were arranged in a complete randomised design with four replications.
Preparation of the germination rolls:
Twenty-one sugar beet seeds were placed at the same distance in three rows on a paper towel (Fig. 1 A). Then the paper towel was rolled. All rolls of one EC level were put in separate dishes (Fig. 1 B) and moistened with 10 ml of the corresponding salt solution.
Fig. 1. Prepared rolled paper towels with placed pelleted sugar beet seeds (A), and rolled paper towels in a dish (B).
At mean room temperature between 22 and 23°C, the dishes were stored in closed glass containers (desiccators with ventilation valves, but without desiccants) to prevent the loss of moisture by evaporation. Because of the large number of seed rolls and the limited space in the desiccators, the experiment had to be carried out in two batches, one after another, where germination and emergence were tested. Each batch required 14 days to be completed. Number of germinated seeds and proportion of emergence were recorded at four different dates: 2, 4, 6, and 10 days after filling and moistening the germination rolls. According to Mavi et al. (2010), seeds were classified as germinated when their radicles reached a length of 2 mm. Emerged plants were classified when they entered the principal growth stage, which starts according to Meier (2001) with the appearance of cotyledons completely unfolded. At the end of each experimental unit, after 14 days, the seedling fresh weight of all seedlings was determined (Fig. 2)

Fig. 2. Emerging sugar beet seedlings in an open rolled paper towel (here untreated seeds) at the end of the germination test.
Percentage of germination was calculated using the following formula:
Where:
GP is germination percentage, SNG is the number of germinated seeds, and SNO is the number of viable seeds (Scott et al., 1984).
Mean germination time (MGT) was calculated using the following formula cited by Ellis and Roberts (1980):
whereby:
N is the number of seeds newly germinated at time D, D is the number of days since beginning of germination test, and F is the final number of germinated seeds.
The emergence percentage was calculated as follow:
Where by:
EMP is emergence percentage, EMS is the number of emerged seedlings and SNO is the number of seeds planted (Mitchell and Vogel, 2012).
The data were statistically analysed using GenStat software.
The germination percentage was not affected significantly by salinity levels, whereas the emergence percentage, mean germination time and seedling fresh weight, were significantly affected by salinity levels as well as by the seed pelleting (Table 1).
Table 1. Mean germination percentage, emergence percentage, mean germination time and seedling fresh weight, of sugar beet seeds as depending on water salinity (EC) and seed pelleting (means of all tested sugar beet cultivars and breeding lines)
Parameters | Germination | Emergence | Mean germination time | Seedlings fresh weight |
Electrical conductivity | ||||
0 | 97.9 | 86.8 | 4.1 | 32.3 |
2 | 97.1 | 89.1 | 4.2 | 41.5 |
4 | 97.3 | 86.8 | 4.6 | 43.2 |
6 | 96.7 | 84.2 | 4.8 | 40.4 |
8 | 96.5 | 81.1 | 5.0 | 37.6 |
10 | 96.1 | 76.1 | 5.2 | 35.8 |
SL | ns | *** | *** | *** |
SE | 0.481 | 0.847 | 0.032 | 0.768 |
LSD | 1.34 | 2.36 | 0.088 | 2.14 |
CV (%) | 3.7 | 7.5 | 5.1 | 14.9 |
Seed treatment | ||||
Pelleted | 96.4 | 73.5 | 4.8 | 34.5 |
Untreated | 97.5 | 94.6 | 4.5 | 42.5 |
SL | ** | *** | *** | *** |
SE | 0.278 | 0.489 | 0.018 | 0.443 |
LSD | 0.774 | 1.36 | 0.051 | 1.24 |
CV % | 3.7 | 7.5 | 5.1 | 14.9 |
SL = significant level (**, *** significant at P levels of 0.01, and 0.001), ns = non- significant, SE = standard error, LSD = least significant difference, CV% = coefficient of variation percentage | ||||
Compared to control (~0 dS/m), seedling emergence percentage was significantly reduced at salinity level 10 dS/m, but the seedling fresh weight was significantly enhanced by all salinity levels (Table 1). Jafarzadeh and Aliasgarazad (2007) reported that both, sugar beet germination and seedling root length, were enhanced by a low salinity level (2 dS/m) in comparison with control (~ 0 dS/m).
The data in Table 1 showed that the fastest mean germination time (4.1 days) in control (0 dS/m) produced the lowest seedling fresh weight (32.3 g/seedling). The slowest mean germination time (5.2 days) due to salinity was observed in the 10 dS/m treatment, which produced lower seedling fresh weight (35.8 g/seedling). This is in accordance with Jamil and Rha (2004), who reported that the required time for germination increased with high levels of salinity. The results in Table 1 show that seed pelleting reduced emergence percentage and seedling fresh weight significantly by 22% and 19%, respectively, but for the germination percentage, the reduction was not remarkable. Kaffka and Hembree (2004) reported that encrusted or film coated seeds emerged significantly slower than untreated seeds. The reason for this could be additional salt stress from the pelleting materials or the pelleting acted as a mechanical obstruction for the seedlings very early first attempts and struggeling for growing up.
The investigated parameters, which include germination percentage, emergence percentage, mean germination time and seedling fresh weight of different sugar beet cultivars and breeding lines are presented in Table 2.
Table 2. Means of germination percentage, emergence percentage, mean germination time and seedling fresh weight, of sugar beet cultivars and breeding lines. Means of all EC levels and naked and pelleted seeds are shown
Parameters | Germination | Emergence | Mean germination time | Seedlings fresh weight |
Cultivars/breeding lines | ||||
Ballade | 96.6 | 93.2 | 4.4 | 43.8 |
ST-SUD-01/14 | 98.2 | 91.8 | 4.6 | 36.5 |
ST-SUD-04/14 | 98.8 | 95.0 | 4.6 | 38.7 |
Lenard | 99.3 | 90.7 | 5.1 | 42.7 |
ST-SUD-05/14 | 97.9 | 71.9 | 4.1 | 34.8 |
ST-SUD-06/14 | 90.5 | 53.0 | 4.9 | 24.6 |
ST-SUD-07/14 | 97.2 | 92.4 | 4.8 | 48.2 |
SL | *** | *** | *** | *** |
SE | 0.520 | 0.915 | 0.034 | 0.830 |
LSD | 1.45 | 2.55 | 0.095 | 2.31 |
CV % | 3.7 | 7.5 | 5.1 | 14.9 |
SL = significant level (*** significant at P levels of 0.001), SE = standard error, LSD = least significant difference, CV% = coefficient of variation percentage | ||||
All tested sugar beet cultivars and breeding lines were characterised by high germination percentages, ranging between 90% and 99%. The two cultivars ‘Ballade’ and ‘Lenard’ as reference varieties were not significantly different from each other in emergence percentage and seedling fresh weight, but ‘Lenard’ had a significantly lower mean germination time than ‘Ballade’. The breeding line ST-SUD-07/14 showed high germination percentage (97%), high emergence percentage (92%), and the highest seedling fresh weight (48 mg/seedling). Moreover, this breeding line had a significantly shorter mean germination time than the cultivar ‘Lenard’, but compared with ‘Ballade’ the germination was slower. The breeding line ST-SUD-06/14 showed the lowest germination percentage, emergence percentage, and seedling fresh weight (Table 2). This indicates that there is still potential for influencing germination time through selection between lines (see also Fig. 4).
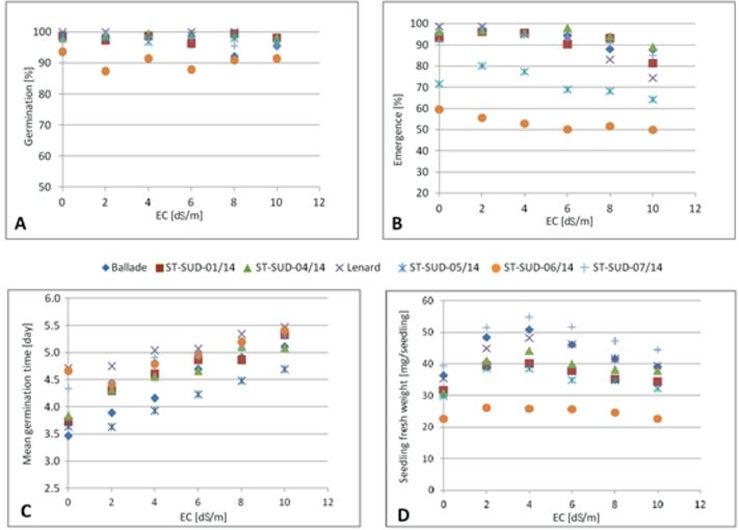
Fig. 4. Effect of water salinity levels (EC dS/m) on (A) germination percentage, (B) emergence percentage, (C) mean germination time and (D) seedling fresh weight of different sugar beet cultivars/breeding lines, averaged over untreated and pelleted seeds.
The effects of salinity on germination percentage, emergence percentage, mean germination time and seedling fresh weight of sugar beet are presented in Fig. 3. The germination percentage decreased linearly with increasing salinity levels. The emergence percentage showed a polynomial trend with increasing salinity levels. The seedling fresh weight initially increased to a salinity level of 4 dS/m, and then decreased significantly, as the salinity level continues to rise up to EC 10 dS/m (polynomial trend). The result is in agreement with Jamil et al. (2006), who reported significantly reduced shoot and root fresh weights in four vegetable species including sugar beet at salinity levels (NaCl) from 4.7 to 14.1 dS/m.
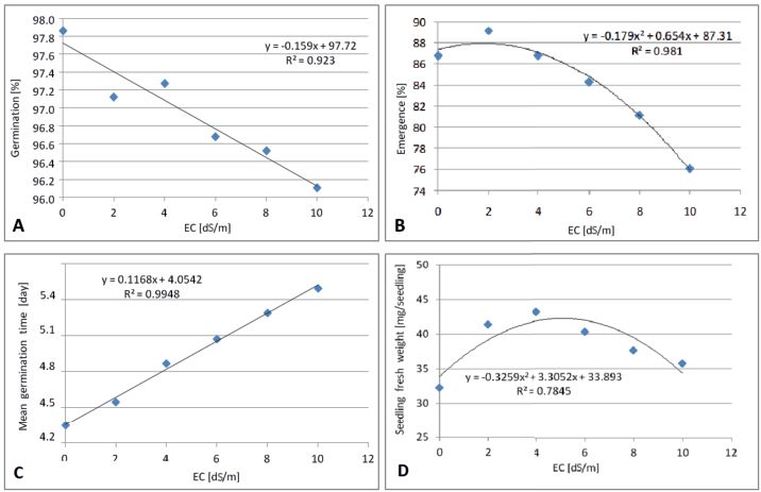
Fig. 3. Effect of different water salinity levels (EC dS/m) on: (A) germination percentage, (B) emergence percentage, (C) mean germination time, and (D) seedling fresh weight of sugar beet. Average of seven cultivars/breeding lines over untreated and pelleted seeds.
Mostafavi (2012) reported on sugar beet and salinity stress and found that all measured traits declined with increasing salinity levels. A linear relationship between the mean germination time and water salinity is shown in Fig. 3 C. The mean germination time increased with increasing salinity levels (R2 = 0.99). The longest mean germination time (5.2 days) was detected in high salinity levels of 10 dS/m (Table 1, Fig. 3 D). It could be concluded that sugar beet react to salt stress in general with delayed germination.
The effects of salinity on sugar beet cultivars and breeding lines are illustrated in Fig. 4 for germination percentage (A), emergence percentage (B), mean germination time (C), and seedling fresh weight (D). A significant effect (p < 0.001) between salinity and sugar beet cultivars/breeding lines was found only for emergence percentage and mean germination time. The data indicated that different cultivars and breeding lines respond differently to salinity under laboratory conditions.
As observed in Fig. 4 A, B, and D, the germination percentage, emergence percentage and seedling fresh weight of different sugar beet cultivars and breeding lines were slightly decreasing with increasing salinity levels, which could be an indication of salt tolerance.
The results reveal that new breeding lines have the potential for improving germination time compared to established cultivars.
The breeding lines ST-SUD-05/14 and ST-SUD-06/14 had a low emergence of 72% and 60% at 0 dS/m, respectively. This could be an indication of low seed quality of these breeding lines, compared to others having a very high emergence percentage ranging from 92% to 99%, indicating high seed quality.
The breeding line ST-SUD-06/14 was inferior to both the other four breeding lines and the two cultivars in germination percentage, emergence percentage, and seedling fresh weight. The breeding line ST-SUD-07/14 showed the highest seedling fresh weights at all tested salinity levels.
The cultivar ‘Ballade’ and the breeding line ST-SUD-05/14 showed a significantly faster mean germination time, while the cultivar ‘Lenard’ and the breeding line ST-SUD-06/14 required more time for germination compared to the others (Fig. 4 D).
In Fig. 5 A, the close relationship (R2 = 0.89) between mean germination time and germination percentage is shown for the six tested water salinity levels. Increasing salinity levels decreased the germination percentage and increased the mean germination time. In the control treatment, the shortest mean germination time (4.1 days) was observed paired with the highest germination percentage (97.9%). Under high salinity stress (EC 10 dS/m), the mean germination time of 5.2 days was the longest, coupled with the lowest germination percentage of 96.1%. Furthermore, there was a reciprocal effect between mean germination time and emergence percentage calculated from the data of the six water salinity levels (Fig. 5 B, R2 = 0.79). This is a typical response of plants to salt stress mainly caused by reduced physiological availability of water or limited nutrient acquisition through antagonistic effects on uptake (e.g. Na+ vs K+). However, this indicates also that there is still potential for influencing germination time through selection between lines (see also Fig. 4). The increasing salinity level decreased the emergence percentage and increased the mean germination time. The mean germination time decreased with increasing emergence percentage. Although the germination time was comparable to that of the control treatment, most seedlings were counted at the lowest salinity level (2 dS/m). The strongest salinity stress resulted in a significantly decreased emergence percentage of 76%.
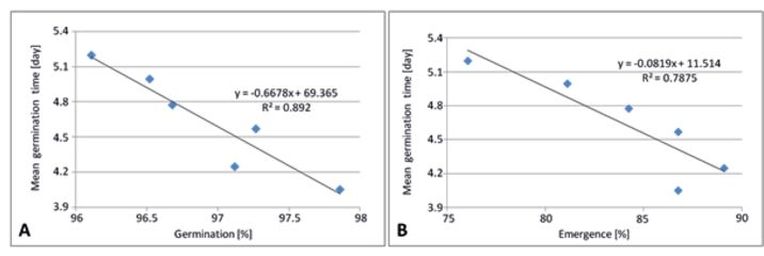
Fig. 5. Relationship between (A) mean germination time and germination percentage as well as (B) mean germination time and emergence percentage of sugar beet cultivars/breeding lines, tested at six salinity levels (EC 0–10 dS/m).
Figure 6 presents the interrelationship between mean germination time and seedling fresh weight (mg/seedling) under unfavourable conditions of stressful salinity ranging between 4.5 and 5.2 dS/m. Both parameters were high correlated with R2 = 0.99. The fast germinating (within 4.5 days) sugar beet seeds produced higher seedling fresh weights (averaged 43 mg/seedling) below a water salinity of 4 dS/m. Salinity stress caused prolonged germination time (maximum 5.2 days) resulting in a lower seedling fresh weights (Fig. 6). In their experiments with pepper Demir et al. (2008) observed also that slow germinating seed lots (high mean germination time) needed a longer time to emerge and produced smaller seedlings.
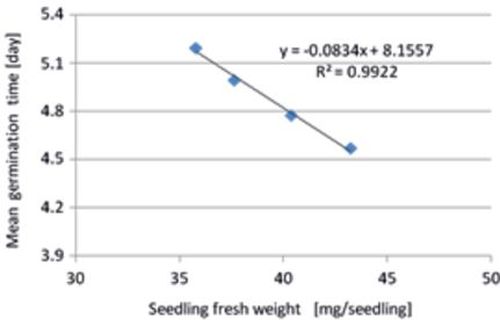
Fig. 6. Relationship between mean germination time and seedling fresh weight of sugar beet cultivars/breeding lines tested at four salinity stress levels (EC 4–10 dS/m).
A positive relationship was observed between the emergence percentage and seedling fresh weights for the two sugar beet cultivars and five breeding lines as indicated in Fig. 7 and Table 2. The breeding line ST-SUD-06/14 that had the lowest emergence percentage (53.0%) produced the lowest seedling fresh weight (24.6 mg/seedling), whereas the group of the others (‘Ballade’, ‘Lenard’, ST-SUD-01/14, ST-SUD-04/14, and ST-SUD 07/14) had higher emergence percentages (90.7%–95.0%) and produced higher seedling fresh weights (36.5–48.2 mg/seedling).
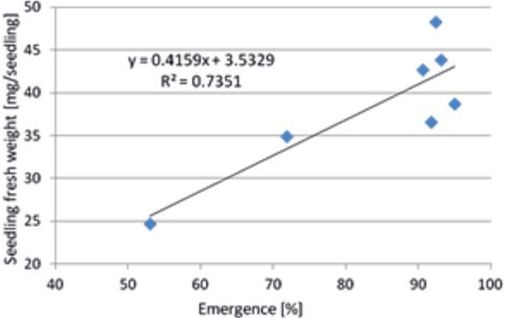
Fig. 7. Relationship between seedling fresh weight and emergence percentage of seven sugar beet cultivars/breeding lines, tested at six levels of salinity (EC 0–10 dS/m).
The findings indicate that the emergence percentage of sugar beet seeds determined by means of germination tests could be a suitable indication to assess the primary seedling growth.
The effects of salinity and seed treatment on different parameters of sugar beet seeds are shown in Fig. 8 A, B, C, and D.
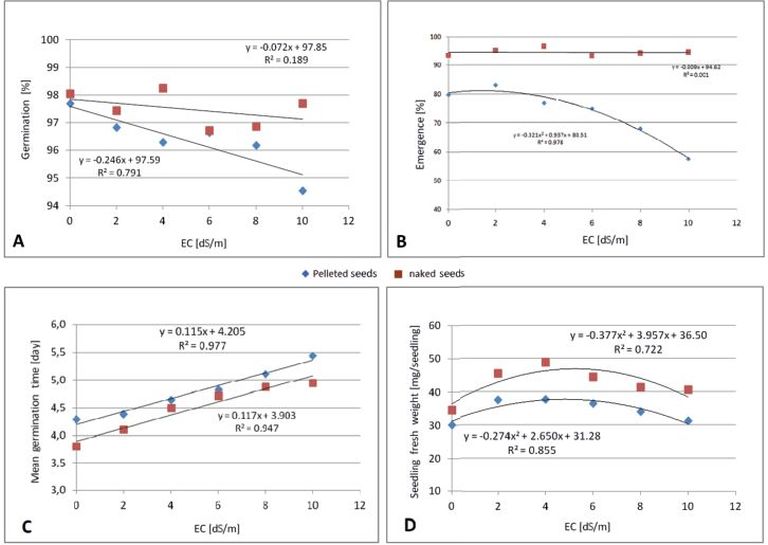
Fig. 8. Effects of different water salinity levels (EC dS/m) and seed treatment on: (A) germination percentage, (B) emergence percentage, (C) mean germination time, and (D) seedling fresh weight of sugar beet cultivars/breeding lines.
The data in Fig. 8 show that there are no interactions between pelleted and untreated seeds in terms of reaction to salinity. Both show a linear reaction to salinity, just on different levels with the pelleted seeds reacting stronger. In contrast, the relation between seedling emergence and seedling fresh weight revealed to follow a polynomial trend (Fig. 8 B and D). As observed from the results, germination percentage and emergence percentage of untreated seeds were nearly unaffected by raising salinity because their correlation coefficients were R2 = 0.189 and R2 = 0.001, respectively (Fig. 8 A and B). In contrast, the germination percentage and emergence percentage of pelleted seeds decreased with increasing salinity (Fig. 8 A and B). It turned out that the untreated seeds were superior to the pelleted seeds in terms of germination percentage, emergence percentage, and seedling fresh weight under the stressful salinity conditions in the laboratory experiment (Fig. 8 A, B, and D). As said before, the reason for this could be additional salt stress from the pelleting materials or that the pelleting acted as a mechanical obstruction for the seedlings very early first attempts and struggling for growth.
Figure 9 shows the effect of the seed treatments on the different germination parameters specified for the individual sugar beet cultivars and breeding lines tested in the experiment. Independent of cultivar and breeding line, seed pelleting generally reduced germination percentage, emergence percentage, and seedling fresh weight.
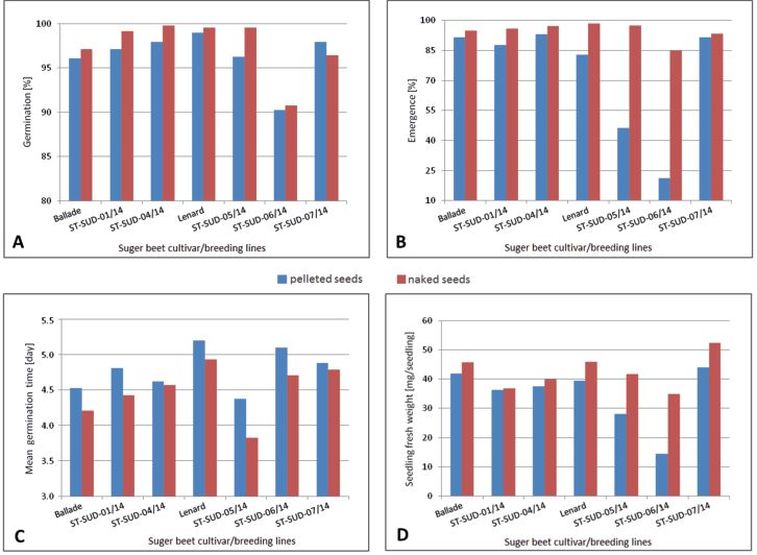
Fig. 9. Comparison of untreated and pelleted seeds of different sugar beet cultivars/breeding lines regarding (A) germination percentage, (B) emergence percentage, (C) mean germination time, and (D) seedling fresh weight, averaged over all salinity levels.
Especially, breeding line ST-SUD-06/14 was significantly (p < 0.001) affected by seed pelleting. The highest reduction in emergence percentage and seedling fresh weight by seed pelleting was recorded with breeding line ST-SUD-06/14 in comparison with the others. The shortest mean germination time was observed for breeding line ST-SUD-05/14, while the cultivar ‘Lenard’ needs the longest mean time to germinate. These results show that there is still enough genetic variability in sugar beet available to direct breeding programs towards altered germination times.
Considering that under field conditions the time for a seedling to emerge is a significant parameter in its competition with weeds for growth resources, such as space, light, water and nutrients and for shortening vulnerable times against pests and diseases, emergence time could be an interesting selection criterion in breeding programmes of sugar beet. The results presented here reveal that even under salinity stress conditions, at least among the lines and varieties investigated in this study, enough genetic variability seems to be available for directing the selection of varieties with faster emergence improving their competitiveness and resistance.
The authors would like to thank the Julius Kühn Institute, the Federal Research Centre for Cultivated Plants – Institute for Crop and Soil Science – in Braunschweig, Germany, for providing the working place to perform this study and the valuable help of co-workers during the experimental time; the Strube Research company for supplying the seeds, and the German Academic Exchange Service (DAAD) for the financial support.
Al-Yahyai, R., S. Al-Ismaily, S.A. Al-Raivahy, 2010: Growing tomatoes under saline field conditions and role of fertilizers. In: A monograph on management of salt-affected soils and water for sustainable agriculture. Sultan Qaboos University, Oman, 83-88.
Blunk, S., A. Hafeez Malik, M. De Heer, T. Ekblad, K. Fredlund, S.J. Mooney, C.J. Sturrock, 2017: Quantification of differences in germination behaviour of pelleted and coated sugar beet seeds using x- ray computed tomography (x- ray CT). Biomedical physics and engineering express 3 (4), DOI: 10.1088/2057-1976/aa7c3f/meta.
Brady, N.C., R.R. Weil, 2002: The nature and properties of soils, 13th ed., Prentice- Hall Inc., New Jersey, USA, 960 pp.
Demir, I., S. Ermis, K. Mavi, S. Matthews, 2008: Mean germination time of pepper seed lots (Capsicum annum L.) predicts size and uniformity of seedlings in germination tests and transplant modules. Seed Science and Technology 36 (1), 21-30, DOI: 10.15258/sst.2008.36.1.02.
Ellis, R.H., E.H. Roberts, 1980: Towards a rational basis for testing seed quality. In: P.D. Hebblethwaite (ed.), Seed Production, Butterworths, London, 605-635.
FAOSTAT, 2018: http://www.fao.org/land-water/databases-and-software/crop-information/sugarbeet/en (accessed 20th August 2018).
Greenway, H., R. Munns, 1980: Mechanisms of salt tolerance in non-halophytes. Annual Review of Plant Physiology 31, 149-190.
Haneklaus, S., E. Bloem, E. Schnug, 2018: Hungry Plants – A Short Treatise on How to Feed Crops under Stress. Agriculture 8 (3), 43, DOI: 10.3390/agriculture8030043.
Jafarzadeh, A., N. Aliasgarazad, 2007: Salinity and salt composition effects on seed germination and root length of four sugar beet cultivars. Biologia 62 (5), 562-564, DOI: 10.2478/s11756-007-0111-7.
Jamil, M., E.S. Rha, 2004: The effect of salinity (NaCl) on the germination and seedling of sugar beet (Beta vulgaris L.) and cabbage (Brassica oleracea L.). Plant resourses 7 (3), 226-232.
Jamil, M., D.B. Lee, K.Y. Jung, M. Ashraf, S.C. Lee, E.S. Rha, 2006: Effect of salt (NaCl) stress on germination and early seedling growth of four vegetative species. Journal of Central European Agriculture 7 (2), 273-282.
Kaffka, S., K. Hembree, 2004: The effects of saline soil, irrigation, and seed treatments on sugarbeet stand establishment. Journal of Sugar Beet Research 41 (3), 61-72.
Khayami, S., R. Tavakka Afshan, S.Y. Sadeghisan, K. Poustini, F. Rouzbeh, Z. Abbasi, 2014: Seed germination, plant establishment and yield of sugarbeet genotypes under salinity stress. Journal of Agricultural Science and Technology 16, 779-790.
Mavi, K., I. Demir, S. Matthews, 2010: Mean germination time estimate the relative emergence of seed lots of three cucurbit crops under stress conditions. Seed Science and Technology 38, 14-25.
Meier, U., 2001: Growth stages of mono-and dicotyledonous plants BBCH Monograph. Federal Biological Research Centre for Agriculture and Forestry, https://www.openagrar.de/receive/openagrar_mods_00036304.
Mitchell, R.B, K., P. Vogel, 2012: Germination and emergence tests for predicting Switchgrass field establishment. Agronomy Journal 104 (2), 458-465, DOI: 10.2134/agronj2011.0168.
Mosta, A.Z., M. Amato, Y.G.M. Galal, A. Hamdi, S.M. Lotfy, 2012: Effects of irrigation with saline water and soil type on germination and seedling growth of sweet maize. Arab Journal of Nuclear Sciences and Applications 45 (2), 537-547.
Mostafavi, K., 2012: Effect of salt stress on germination and early seedling growth stage of sugar beet cultivars. American-Eurasion Journal of Sustainable Agriculture 6 (2), 120-125.
Podmore, C., 2009: Irrigation salinity – Causes and impacts. Primefacts for profitable, adaptive and sustainable primary industries, Primefact 937, NSW Government, Industry & Investment, Wagga Wagga. http://www.dpi.nsw.gov.au/GGTSPU-555dc3ff26f53e90-290-2165946-z0rRqjS0WyKFAf4r-LOD/__data/assets/pdf_file/0018/310365/Irrigation-salinity-causes-and-impacts.pdf (accessed 8th September 2017).
Scott, S.J., R.A. Jones, W.A. Williams, 1984: Reviews of data analysis methods for seed germination. Crop Science 24, 1192-1199.
Shannon, M.C., 1998: Adaptation of plants to salinity. Advances in Agronomy 60, 75-119.
Yadav, S., M. Irfan, A. Ahmad, S. Hayat, 2011: Causes of salinity and plant manifestation to salt stress: A review. Journal of Environmental Biology 32, 667-685.