

Influence of arbuscular mycorrhiza fungi, rice-husk-drived biochar and compost on dry matter yield, nutrients uptake and secondary metabolites responses of Iranian borage Echium amoenum Fisch & C. A. Mey
Einfluss vesikulär-arbuskulärer Mykorrhiza, Biochar aus Resisspreu und Biokompost auf Ertrag, Nährstoffaufnahme und sekundäre Inhaltstoffe von iranischem Gurkenkraut (Echium amoneum; Fisch & C. A. Mey)
Journal für Kulturpflanzen, 70 (12). S. 329–341, 2018, ISSN 1867-0911, DOI: 10.1399/JfK.2018.12.01, Verlag Eugen Ulmer KG, Stuttgart
This study was carried out to investigate the effect of bio-fertilizers including mycorrhiza (MY), rice husk compost (RHC), and biochar (RHB) on dry matter yield, nutrients uptake and some secondary metabolites of the medicinal plant Echium amoenum Fisch & C. A. Mey. The experiment was conducted in a completely randomized design and executed with six treatments and six replications. Treatments comprised of T1: control, T2: MY, T3: RHC, T4: RHB, T5: RHC+MY and T6: RHB+MY. The following parameters were studied: leaf dry weight, macro and micro nutrient uptake, chlorophyll a, chlorophyll b, carotenoids, proline, anthocyanin, flavonoid, mucilage and carbohydrate content. The results show that application of RHC, RHB and MY individually or in combination significantly affected the studied parameters in comparison with the control treatment. In all cases, combined application of bio-fertilizers together with mycorrhiza application (T5 and T6) had a more positive impact on the studied parameters compared to the application of each treatment alone.
Key words: Bio-fertilizers, Nutrient uptake, Dry matter yield, Secondary metabolites
In einem Gewächshausversuch wurde der Einfluss von Bio-Düngern wie vesikulär-arbuskulärer Mykorrhiza, Biochar aus Reisspreu und Biokompost auf Ertrag, Nährstoffaufnahme und sekundäre Inhaltstoffe der Medizinalpflanze Echium amoneum; Fisch & C. A. Mey (iranisches Gurkenkraut) geprüft. Die Varianten waren komplett randomisiert. Alle Behandlungen zeigten signifikante Effekte auf Trockenmasse, Nährstoffaufnahme und Gehalte an Chlorophyll, Carotinoiden, Prolin, Anthocyanen, Flavonoiden, Schleimstoffen und Kohlenhydraten.
Stichwörter: Bio-Dünger, Nährstoffaufnahme, Sekundärstoffwechsel, Biochar, Gurkenkraut
Nowadays, one third of human demands for medicines is obtained from plants (Agatonovic-Kustrin et al., 2015). Medicinal plants are used for different purposes. In general, products derived from secondary metabolites are responsible for the impact of medicinal plants. (Cabello et al., 2005). Medicinal plants cultivation has been increased during the last two decades across the world (Salehi et al., 2016) because of their great importance in modern and traditional medicine. They are also used as raw materials for pharmaceutical, cosmetic and fragrance industries (Karthikeyan et al., 2009). The amount of effective ingredients and chemical characteristics of medicinal plants is highly depended on the yield of these plants and is also affected by the nutritional status and fertility of the soils (Raei and Alami-Milani, 2014). A fertile soil must contain the required amounts and an optimal combination of minerals, organic, macro and micro elements needed for these plants (Karlen et al., 1997). Environmental impacts of chemical fertilizers and also expenses of their production lead to increase in tendering toward application of bio-fertilizers (Kannayan, 2002). The use of bio-fertilizers has long been recognized as an effective measure of improving the structure and fertility of the soils (Follet et al., 1981). The content of soil organic matter is generally one of the most important criteria of soil quality and is influencing the processes occurring in the soil and many soil properties (Cercioglu et al., 2014). Adding compost to agricultural soils, as a soil conditioner and an organic fertilizer has beneficial effects on crop development and yield by improving soil physicochemical and biological properties (Agegnehu et al., 2016; Cercioglu et al., 2014). There are numerous studies illustrating that using organic matter has a positive effect in cultivation of medicinal plants (Raei and Alami-Milani, 2014). The conducted research showed a significant improvement in yield and chemical composition of medicinal plants grown on soils treated with compost (Hendawy and Khalid, 2011). Also, biochar as an organic substrate has drawn a lot of international attention as a useful organic material, which can improve the status of soil fertility (Lehmann et al., 2006). It can enhance and improve the physical and chemical properties of soils, increase microbial activity, reduce nutrient leaching, and alter nutrient availability, thus contributing to improve plant growth conditions (Egamberdieva et al., 2016). Studies on medicinal plants indicate that application of biochar not only promotes growth of these plants but also significantly affects plant chemical compositions via an enhancement in plant nutritional status (Amei et al., 2016). One further method in organic cultivation of medicinal plants is mycorrhization. Mycorrhizal fungi are beneficial microorganisms and hence, have been considered as bio-fertilizer (Raei and Alami-Milani, 2014). The improved productivity of inoculated plants was attributed to enhanced uptake of immobile nutrients such as phosphorus, zinc and copper (Karthikeyan et al., 2009). Results of different studies have documented that mycorrhizal inoculation caused an increase in yield of chemical compositions value of medicinal plants in comparison with non-inoculated plants. In fact, also observed improvements of secondary metabolites may be due to an enhancement of nutrients uptake caused by mycorrhization (Zolfaghari et al., 2013; Kumar et al., 2015). Iranian borage (Echium amoenum Fisch & C. A. Mey.) from the plant family Boraginaceae is a wild annual herb and known as a valuable medicinal plant native to Iran and Syria and was therefore selected for this experiment (Mehrbani et al., 2005). The usable parts of borage (as medicinal plant) are petals and leaves which have been advocated for a variety of effects such as demulcent, anti-inflammatory and analgesic, especially for common cold, anxiolytic, sedative and other psychiatric symptoms (Morteza-Semnani and Saeedi, 2013). The leaves are glazed and contain a lot of vitamin C. The brewed leaves are useful for respiratory tract discomforts. The leaves can be used either raw in the salad or cooked like spinach (MIRAJ and KIANI, 2016). To increase knowledge on the impact of mycorrhization and organic substrates on medicinal plants this study was aimed to determine the effect of rice husk compost, rice husk biochar and mycorrhization on nutrients status and chemical composition including some secondary metabolites of Iranian borage of E. amoenum.
A completely randomized experimental design was carried out with six treatments and six replications in order to investigate the effect of rice husk compost (RHC), rice husk biochar (RHB) and mycorrhiza (MY) on dry matter yield, nutritional status and some secondary metabolites of E. amoenum. The experiment was carried out in a greenhouse located at the college of agriculture, Shiraz University, Iran from January to August 2017. The daily temperature was 25–27°C and 16–17°C during the night. A silty loam soil was collected from the top layer (0–30 cm) of a field located at the experimental station of the college of agriculture. RHB was prepared from the rice husk provided from the experimental station using a laboratory furnace under limited oxygen supply at 600°C for 4 h. Previous studies indicated that a procedure at higher temperatures resulted in a higher C content (Liang et al., 2016). In addition, with increasing temperature the ash and fixed C contents increased but a reduction in the content of volatile materials was observed (TAG et al., 2016). To provide RHC, rice husk was composted under aerobic conditions in the greenhouse for 2 months. Claroideoglomus etunicatum* was used to create a mycorrhizal system. Treatments comprised of T1: control, T2: MY, T3: RHC, T4: RHB, T5: RHC + MY and T6: RHB + MY. Five kg pots were used and filled by treated soil. 1% organic matter (OM) was applied for both RHC and RHB (50 g OM per 5 kg soil).
Mycorrhizal infection was done by adding 100 g of C. etunicatum inoculum (800 spores, root colonization of 75%) into the top layer of soil (3–5 cm) before sowing. Two seeds were planted into the pots. Plants were irrigated by distilled water daily and the required nutrients were added based on the soil testing results (150 mg/kg N derived by urea, 5 mg/kg Fe derived by sequoestrine, 5 mg/kg Zn derived by Zn2So4 and 2.5 mg/kg Cu derived by Cu2So4). Some of the soil and organic substrates properties are given in Table 1 and 2. Plants were harvested 7 months after emergence and shoots and roots were separated and were cleaned by dipping them in water 2 to 3 times till the adhering soil particles were removed. To perform plant analysis, the leaf samples were transported into the laboratory and were dried in an oven at 65°C for 48 h. To measure mucilage and flavonoid content in leaf material, the powdered samples were needed. The investigated plant parameters comprised of the dry matter yield of plant leaves, plant nutrition analysis and some secondary metabolites. Also a chinese mountaineer was used to grind the fresh sample for measuring chlorophyll pigments, proline, anthocyanin and carbohydrate.
Table 1. Some physicochemical properties of the examined soil (0–30 cm)
Parameters | ||||||||||||||
Texture | Sand | Silt | Clay | pH | EC | OM | CCE | N (T) | P (A) | K (A) | Fe (A) | Zn (A) | Cu (A) | Mn (A) |
Silt Loam | 32.94 | 50.24 | 16.82 | 7.72 | 0.69 | 1.2 | 42.8 | 0.143 | 15.4 | 446.54 | 4.99 | 0.73 | 1.79 | 9.6 |
Unit | ||||||||||||||
– | % | % | % | – | dS m-1 | % | % | % | mg kg-1 | mg kg-1 | mg kg-1 | mg kg-1 | mg kg-1 | mg kg-1 |
T: Total value, A: Available value, EC: Electrical Conductivity, OM: Organic Matter and CCE: Calcium Carbonate Equivalent | ||||||||||||||
Table 2. Some properties of the applied organic fertilizers
Parameters | |||||||||||
pH | EC | OM | N (T) | P (T) | K (T) | Fe (T) | Zn (T) | Cu (T) | Mn (T) | Ash | |
RHC | 6.77 | 2.13 | 11.4 | 0.653 | 0.07 | 0.29 | 1976 | 31.6 | 14.4 | 132 | 25 |
RHB | 6.85 | 2.25 | 24.3 | 0.965 | 0.255 | 1.45 | 5636 | 63.7 | 20.2 | 201 | 44 |
Unit | |||||||||||
– | dS m–1 | % | % | % | % | mg kg–1 | mg kg–1 | mg kg–1 | mg kg–1 | % | |
RHC: rice husk compost, RHB: rice husk biochar and T: Total value. | |||||||||||
The biomass of leaves was washed thoroughly to remove all the adhering soil particles in running tap water and gently pressed in folds of filter paper to remove excess moisture. The samples were wrapped in paper and placed in hot air oven at 65°C for 48 h. After 48 h, the plant parts were removed, cooled in desiccators and weighed to record dry weight.
To measure P, K, Fe, Zn, Cu and Mn, the powdered samples were needed to be ashed so they were combusted in a muffle furnace at 550°C over 2 h. EC and pH of compost and biochar were determined in a 1:10 ratio of water and organic matter (Gillman and Sumpter, 1986). Fe, Zn, Cu and Mn contents of the plant samples, RHC and RHB were estimated by dry ashing and dissolving the ashed samples in 2N HCl and using atomic absorption (Chapman and Pratt, 1961). Total concentration of N in plant samples, RHC and RHB were measured by Kjeldahl (Bremner, 1996). Total P in plant samples, RHC and RHB were determined by Vanadate-Molybdate yellow method, where phosphate ions react with vanadate-molybdate reagent to form a yellow dye. The measurement wavelength was 435 nm using a spectrophotometer (Chapman and Pratt, 1961). Total K was determined by the flame photometer in the extraction of the dry ash method (Chapman and Pratt, 1961). Ash content of RHB and RHC was measured by standard ASTMD-2866 method on weight basis, where 1.0 g of oven-dried RHB and RHC was briefly heated at 600°C overnight, cooled and weighed again (Rajkovich et al., 2012).
One gram of fresh leaf sample was finely cut and gently mixed with a clean pestle and mortar. To this homogenized leaf material, 20 ml of 80% acetone and 0.5 g MgCO3 powder was added. The materials were further grind gently. The sample was then put into a refrigerator at 4°C for 4 hours. Thereafter, the sample was centrifuged at 500 rpm**for 5 min. The supernatant was transferred to 100 ml volumetric flak. The final volume was made up to 100 ml with addition of 80% acetone. The color absorbance of the solution was estimated by a spectrophotometer using 645, 663 and 470 nm wavelength against the solvent. Acetone (80%) was used as a blank. The solution mixture was analyzed for chlorophyll a, chlorophyll b and carotenoids content in the spectrophotometer. The pigments were expressed as mg per g fresh leaf (Porra et al., 1989). The equation used for the quantification of chlorophyll a, chlorophyll b, and carotenoids are:
Chl a = (11.75×A 662.6) – (2.35×A 645.6)
Chl b = (18.61×A 645.6) – (3.96× A 662.6)
Carotenoids = (1000 A 470 – 1.82 Chl a –85.02 Chl b)/198
“A” is absorbance by spectrophotometer.
Proline accumulation was determined by extracting fresh samples in 3% sulfosalicylic acid. The extract was heated in a water bath for 10 min and then filtered through filter paper. Two ml of the extract was mixed into 6 ml assay media containing 2 ml ninhydrin solution and 2 ml acetic acid. After that, all samples were incubated at 100°C for 30 min and cooled at room temperature. The colored product was extracted by adding 4 ml toluene. Finally, absorbance of organic layer was measured at 520 nm. Toluene was used as blank. Amount of proline was reported as micromole per g fresh weight (μmol g–1 fw) (Bates et al., 1973).
Where M is proline content obtained from the standard curve, T is 4 ml of the added toluene, and W is weight of fresh leaf (0.5 g).
Wanger method (1979) was used to measure the amount of anthocyanin in leaves. 0.5 g of fresh leaf was weighted and completely ground in porcelain mortar with some amount of acidic methanol (methanol and hydrochloric acid in a ratio of 99:1%) and the extract was poured into test tubes and put in the dark for 24 h at 25°C. Then the extract was centrifuged for 10 min at 4000 g and the supernatant absorbance was read using a spectrophotometer at 550 nm wavelength. The anthocyanin content was calculated using the following formula:
A = ε b c
Where A is absorbance, ε is molar absorptivity, b is width of the measurement cell and equals to one centimeter and c is concentration in units of molarity.
The aluminum chloride colorimetric method was used to estimate the flavonoids. Dried leaf samples of the plant (10 mg) was extracted by 80% ethanol then a definite volume of solution (0.5 ml) was mixed with 1.5 ml of 95% ethanol, 0.1 ml of 10% aluminum chloride, 0.1 ml of 1 M potassium acetate and 2.8 ml of distilled water. After incubation at room temperature for 30 minutes, the absorbance of the reaction mixture was measured at 415 nm with a UV–VIS spectrophotometer. The amount of 10% aluminum chloride was substituted by the same amount of distilled water in blank. Quercetin was used to make the calibration curve, the reacted flavonoids of the extracts with aluminum chloride was used for the determination of flavonoids content as mg g–1 dry weight of plant leaves (CHANG et al., 2002).
Mucilage extraction was performed by hot extraction method. Briefly, 2 g of dry samples mixed with 10 ml of acidified distilled water (pH = 3.7). Then, 200 ml of distilled water (the same pH as above) was added and blended for 20 min. After separating the waste products using a Buchner funnel, the remaining solution was centrifuged and ethanol 96% was added (4 times the solution volume). The final solution kept at 4°C for 24 h to mucilage precipitation. The precipitate was separated by vacuum filtration using a Buchner funnel and then weighted after drying. The content of mucilage was reported in percent (%) (Hirst and Jones, 1955).
To measure soluble carbohydrate 0.2 g of the leaf fresh tissue with 10 ml of 95% ethanol were put in hot water bath at 80°C for 60 min. One ml of extract was reacted with 1 ml of 0.5% phenol and 5 ml of 98% sulfuric acid. The absorption of each sample was measured at 483 nm with a UV–VIS spectrophotometer. The concentration was determined from a standard table and calculated on a fresh weight basis (μg glucose g–1 fw) (Dubois et al., 1956).
The statistical analysis was performed by the SPSS software and comparison between applied means was done using Duncan’s multiple range tests (DMRT) at the 5% significant level. The figures were drawn by the MS Ecxel software.
Combined or individual application of RHC, RHB and MY significantly increased dry matter yield of Echium leaves compared to the control (P ≤ 0.05). The highest value of leaf dry matter belonged to the RHB + MY treatment (33.2 g pot–1) but was not significantly different from RHC + MY treatment (Fig. 1). Improvement in crop performances are consistent with other findings, which may be attributed to improved availability of nutrients and soil moisture content (Zhang et al., 2016). Based on the data given in Table 3., there was a positive correlation between macro and micronutrient uptake and plant dry matter yield. In addition another experiment showed that organic fertilizers significantly influenced leaf dry matter content, which might be due to the high concentration of nutrients in organic fertilizers (Hosseini Vakili et al., 2015). Further results proved that due to high levels of nutrients, especially nitrogen in organic amendments, vegetative growth and dry matter yield of medicinal plant Plantago psyllium increased (Yadav et al., 2003). Compost treatment significantly increased herb dry matter yield via beneficial effects on soil physicochemical and biological properties and improving nutrients availability (Abdullah et al., 2012). Furthermore, it was described that increased in plant growth and dry matter yield as a result of biochar additions can be attributed to optimization of the availability of plant nutrients (Agegnehu et al., 2016). Based on the results of the present research mycorrhization increased dry matter which is in agreement with those results obtained by other researchers. For example, mycorrhization had a significant effect on dry mass production of Plantago japonica in comparison with non-mycorrhizal treatments (Higo et al., 2010). Similarly, Selvakumar and Thamizhiniyan (2011) illustrated that mycorrhization significantly improved dry matter of Capsicum annuum L., which may be reasoned by the important efficacy of mycorrhization on nutrients availability enhancing growth and biomass. In conformity of our findings Swamy et al. (2016) stated that there was an increase in plant growth and dry matter of medicinal plants inoculated with mycorrhiza compared to non-inoculated plants due to improvement of nutrient uptake.

Fig. 1. Effect of bio fertilizers on leaf dry matter yield content of Echium; Different letters indicate statistical differences between groups according to DMRT at P ≤ 0.05. T1: Control, T2: MY, T3: RHC, T4: RHB, T5: RHC + MY and T6: RHB + MY.
Table 3. Correlation among nutrients uptake, dry matter and secondary metabolites of Echium
N | P | K | Fe | Zn | Cu | Mn | Mcy | Antcy | Chl a | Chl b | Crts | Pro | Carbo | Flvo | Rdw | Ldw | |
N | .429ns | .846** | .851** | -.035* | .834** | .860** | .852** | .768** | .964** | .913** | .949** | -.777** | .721** | .983** | .848** | .848** | |
P | .525* | .547* | -.059* | .548* | .519* | .610** | .468ns | .416ns | .455ns | .487ns | -.520* | .502* | .495* | .513* | .579* | ||
K | .974** | .101ns | .991** | .988** | .962** | .864** | .837** | .809** | .867** | -.947** | .911** | .869** | .966** | .940** | |||
Fe | .044* | .969** | .992** | .984** | .997** | .965** | .921** | .972** | -.875** | .823** | .906** | .997** | .987** | ||||
Zn | . 521 * | . 741* | .511* | .597* | .512** | . 542* | .512** | -.533* | .517* | . 611* | .563* | .519* | |||||
Cu | .983** | .961** | .973** | .746** | .734** | .701** | -.964** | .915** | .877** | .956** | .932** | ||||||
Mn | .973** | .933** | .909** | .866** | .813** | -.909** | .865** | .839** | .991** | .969** | |||||||
Mcy | .882** | .843** | .813** | .773** | -.883** | .844** | .903** | .973** | .985** | ||||||||
Antcy | .982** | .980** | .949** | -.987** | .952** | .895** | .882** | .833** | |||||||||
Chl a | .967** | .921** | -.975** | .942** | .882** | .854** | .795** | ||||||||||
Chl b | .980* | -.979** | .929** | .870** | .798** | .747** | |||||||||||
Crts | -.960** | .911** | .839* | .740* | .694** | ||||||||||||
Pro | -.971** | -.993* | -.851** | -.818** | |||||||||||||
Carbo | .867** | .800** | .774** | ||||||||||||||
Flvo | .890** | .850** | |||||||||||||||
Rdw | .985** | ||||||||||||||||
**. Correlation is significant at the 0.01 level, *. Correlation is significant at the 0.05 level, ns. Correlation is not significant, Mcy: Mucilage, Antcy: Anthocyanin, Chl a: Chlorophyll a, Chl b: Chlorophyll b, Crts: Carotenoids, Pro: Prolin Flvo: Flavonoid, Rdw: Root dry weight, Ldw: Leaf dry weight. | |||||||||||||||||
Plant uptake of N, P, and K was significantly affected by separate or combined application of bio-fertilizers (P ≤ 0.05). The highest values were observed in co-application of RHB + MY. Maximum N, P and K uptake in plant were 591.8 58.1 and 620.5 mg pot–1, respectively (Table 4). These findings are in agreement with others reported that bio-fertilizers are able to enhance plant macronutrient uptake (Markus et al., 2008). Zhang et al. (2016), documented that separate or combined application of biochar and compost had a significant influence on plant N, P and K uptake in comparison with inorganic amendments due to the impact of biochar on increasing fertilizer use efficiency and also due to a temporary change in pH which is useful for uptake of lower available nutrients (Amei et al., 2016). It was revealed by Heitkotter and Marschner (2015), that the chemistry of biochar can lead to the retention of K by cation exchange capacity associated with acidic functional groups formed during oxidation process on biochar surfaces, hence, K can be more available for plant. Following organic matter application in soil, more availability of N, P and K was observed for plants in comparison with mineral fertilizer treated plants, which was due to the fact that compost and biochar supplied these nutrients into soil and also improved their availability for plants uptake by reducing the sorption and leaching potential of nutrients (Agegnehu et al., 2015). Additionally, Subramanium et al. (2018) stated that improved status of N uptake in soil induced by organic matter application may be because of more reduction of organic matter compared to the NH3 loss, which usually enhances N uptake. They also reported that the improvement of P and K uptake in plants following compost application was due to the mineralization. Our results are in agreement with other research reports which described that the positive effect of mycorrhization on plant macronutrients uptake is due to the ability of mycorrhiza to translocate nutrients via extending hyphae and increasing root absorbing surface (Zhang et al., 2016). The same results were obtained for micronutrient uptake in plants. Micronutrient uptake in plants was affected by separate or combined application of treatments (P ≤ 0.05). The maximum amounts of micronutrient uptake resulted from co-application of RHB + MY or RHC + MY. The highest values of Fe, Zn, Cu and Mn uptake in plant were 6.90, 0.85, 0.24 and 1.02 mg pot–1, respectively (Table 4). Similar to the other studies` results, through the present experiment there was an enhanced plant essential elements uptake as a result of bio-fertilizers application in soil (Allah Wadhayo et al., 2015). More plant nutrient uptake, particularly microelements, may be due to the organic acids provided as a decomposition product of organic matters (Conversa et al., 2015). With respect to the influence of mycorrhization, our findings are in agreement with that observed by other researchers who reported improved plant nutrient uptake following mycorrhization (Neumann et al., 2009). One reason for high nutrient uptake resulted by mycorrhization might be due to the ability of mycorrhiza to colonize roots completely, which enhances nutrients content by the extraradical mycelium (Kumar et al., 2015). The same conclusion was reported by Briccoli et al. (2015), who studied the effect of mycorrhization and documented an increase in the percentage of Mn translocation to the leaves, from 14 to 22%, which also led to increase photosynthetic activity in the mycorrhizal plants, since Mn is a constitutive element of photosystem II (Enami et al., 2008). Since biochar and compost are derived from biomass, they have high amounts of macronutrients (N, P, and K), and micronutrients (Fe, Zn, Cu, and Mn) and enhance nutrient uptake by plants (Agegnehu et al., 2015). Considering this and the results of chemical analysis of compost and biochar in the present study, an enhanced nutrient uptake of plants could be expected.
Table 4. Effect of bio fertilizers on uptake of nutritional elements of the leaf
Micronutrients (mg pot–1) | ||||
Fe | Zn | Cu | Mn | |
Control | 4.29 d | 0.508 d | 0.138 d | 0.669 d |
MY | 5.17 bc | 0.608 c | 0.164 c | 0.779 c |
RHC | 5.72 b | 0.652 bc | 0.189 b | 0.877 b |
RHB | 5.70 b | 0.676 bc | 0.192 b | 0.888 ab |
RHC* MY | 6.90 a | 0.802 b | 0.228 a | 1.008 a |
RHB* MY | 6.90 a | 0.853 a | 0.239 a | 1.020 a |
Macronutrients (mg pot–1) | ||||
N | P | K | ||
Control | 373 d | 27.7 d | 383 d | |
MY | 468. bc | 45.5 b | 482 c | |
RHC | 475 bc | 39.9 bc | 575 b | |
RHB | 523 b | 40.6 bc | 596 b | |
RHC* MY | 561 ab | 59.6 a | 639 a | |
RHB* MY | 592 a | 58.1 a | 620 a | |
Means within the same column followed by the same letter indicate non-significant difference according to DMRT (P ≤ 0.05). MY: mycorrhization, RHC: rice husk compost and RHB: rice husk biochar | ||||
As expected, addition of organic bio-fertilizers had a positive and significant effect on chlorophyll pigments content including chlorophyll a (Chl a), chlorophyll b (Chl b) and carotenoids in comparison with the control (P ≤ 0.01). In case of these parameters the highest values were related to the combination treatments, too. The maximum amount of Chl a, Chl b and carotenoids was observed in combined treatments RHB + MY and were 7.75, 5.85 and 4.90 mg g–1 fw, respectively (Table 5). It has been found that the higher biomass resulted from organic fertilizer addition is because of the positive effects of bio-fertilizer application on chlorophyll pigments content (Agegnehu et al., 2015). As the results of present study showed, there was a positive correlation between N uptake and chlorophyll pigment content; besides N, uptake of other nutrients including K, Fe, Cu, and Mn had a positive correlation with chlorophyll pigments content, too (Table 3). A study conducted by Karanatsidis and Berova (2009) showed that organic N fertilizers significantly influenced the content of Chl a, Chl b and carotenoids because of the fact that nitrogen is a structural element of chlorophyll and affects its formation and accumulation. Furthermore chlorophyll pigments in Matricaria chamomilla L. were found to be significantly increased after vermicompost application in soil in the study of Salehi et al. (2016). According to previous findings, there is a significant correlation between leaf N content and chlorophyll concentration. Leaf chlorophyll content is a good indicator to determine the N status in plants. In general, it can be supposed that vermicompost as an organic fertilizer can increase chlorophyll and carotenoid contents by increasing the amount of N availability for plants. By this, more sunlight can be absorbed, more assimilates can be produced and plant growth and yields can finally be enhanced (Feng et al., 2015). As a result of a carried out experiment, it was documented that inoculating plants with Gigaspora margarita*** led to higher leaf chlorophyll concentrations in non-inoculated plants and chlorophyll pigments concentration significantly increased (Zuccarini, 2007). The reason may be due to the increase in photosynthesis or presence of larger bundle sheath chloroplasts present in inoculated leaves (Devi and Reddy, 2004).
Table 5. The effect of bio fertilizers on the content of chlorophylls and carotenoids (mg g–1 fw)
Chlorophyll a | Chlorophyll b | Carotenoids | |
Control | 6.24 d | 4.54 d | 3.70 d |
MY | 6.20 c | 4.96 c | 4.04 c |
RHC | 6.79 bc | 5.08 bc | 4.29 b |
RHB | 7.16 b | 5.30 b | 4.32 b |
RHC + MY | 7.51 ab | 5.80 a | 4.84 a |
RHB + MY | 7.75 a | 5.85 a | 4.90 a |
Means within the same column followed by the same letter indicate non-significant difference according to DMRT (P ≤ 0.05). MY: mycorrhization, RHC: rice husk compost and RHB: rice husk biochar. | |||
Data in Fig. 2 show that application of bio-fertilizers separate or combined with mycorrhiza significantly decreased the concentration of proline in comparison with control (P ≤ 0.05). The highest value observed in control was 5.21 μmol g–1 fw, while the lowest proline content belonged to the combined treatment of RHB + MY and was 3.11 μmol g–1 fw. Proline, an amino acid, plays an important role in plants and is essential for plants metabolism (Xue et al., 2009). Numerous studies have shown that the proline content in plants increases under different environmental conditions such as drought, high salinity, heavy metals and oxidative stress (Zahed Chkovari et al., 2015). Proline protects plants from various stresses and helps plants to recover from stress more rapidly via different strategies like affecting plant-water relations followed by maintaining turgidity of cells under stress or increasing the rate of photosynthesis (Shamsul et al., 2012). Regarding to the described results of this experiment with respect to the plant dry matter yield and chlorophyll pigments content, it is clear that bio fertilizers application improved soil physicochemical and biological conditions, enhanced water and nutrient uptake rate and generally decreased in stress conditions. Data shown in Table 3 also revealed that there was a negative correlation among nutrients uptake and chlorophyll pigments with leaf proline concentration. So as expected, the plant proline content decreased by improving soil conditions in terms of both physicochemical and biological properties. With regard to the effect of compost application on proline contents, it was concluded that proline content in Jatropha curcas was reduced by compost addition, which is attributed to the ability of applied compost into the soil to retain plant nutrients that are otherwise washed out (Mazhar et al., 2011). Moreover, Maie Mohsen et al. (2016) illustrated that plants fertilized with vermicompost as an organic matter, had the lowest values of proline content (4.99 and 4.48 mg g–1 fw) compared to unfertilized control plants giving the highest values (9.60 and 8.73 mg g–1 fw). In conformity to our results, it also was documented that mycorrhization led to a decrease in proline content in C. annuum L. (Ruscitti et al., 2011). Our findings are consistent with other studies observing that proline concentrations decreased under non-stress conditions followed by organic and bio-fertilizer application into the soil (Yang et al., 2000; Nanjo et al., 2003).
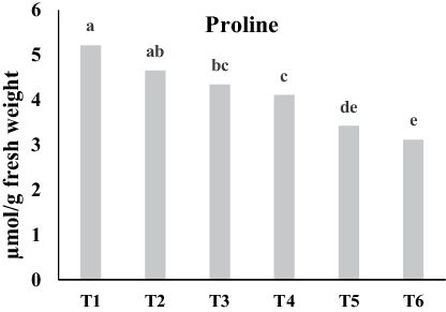
Fig. 2. Effect of bio-fertilizers on leaf proline content. Different letters indicate statistical differences between groups according to DMRT at P ≤ 0.05. T1: Control, T2: MY, T3: RHC, T4: RHB, T5: RHC + MY and T6; RHB + MY.
Separate or combined application of different bio-fertilizers show a significant effect on the anthocyanin content of plant leaves (P ≤ 0.05). The lowest (4.64%) and the highest (6.39%) values of anthocyanin were observed in control and combined treatment of RHB + MY, respectively (Fig. 3). Application of MY, RHC and RHB individually increased leaf anthocyanin content compared to the control with the percentage of 29.9, 20.0% and 23.0% respectively. However, combined application of bio-fertilizers was more effective and increased the anthocyanin content by 47.0% and 47.1% in RHC + MY and RHB + MY, respectively. Anthocyanins are plant pigments which play relevant roles in plant propagation, plant defense mechanisms, color of fruits and vegetables and there are also evidences proving their benefits in human health (Santos-Buelga et al., 2014). Enhanced nutrient uptake following bio-fertilizer application affect anthocyanin concentration in plant (Shaalan, 2005). The results of the present study demonstrated that the increase in nutrient uptake following bio-fertilizer application had a positive correlation with anthocyanin content in plant (Table 3). It was reported that treated plants with organic fertilizers led to a significant increase in anthocyanin content compared with untreated plants (Elham and Rania, 2015). N-fertilizer application cause to an increase in N uptake by plants and also enhance in expression of the responsible gene for anthocyanin synthesis, hence, it can be expected that anthocyanin content of plant is affected by N-fertilizer application (Yamuangmorn et al., 2018). Considering RHC and RHB as organic-N resources and the role of mycorrhiza in nitrogen availability for plant, it can be concluded that the increased anthocyanin concentration followed by bio-fertilizer application in the present study may be caused by an enhancement in nitrogen uptake rate. Moreover, plant nutritional status especially nitrogen has an important effect on the control of anthocyanin concentrations (Dorais, et al., 2008). In conformity with our results, Lingua et al. (2013) reported that any inoculation with mycorrhiza increases the concentration of anthocyanin in plant. LU et al. (2015) illustrated that mycorrhization significantly boosted anthocyanin accumulation in Dioscorea spp, compared with the control. Indeed, mycorrhization is considered as a kind of microbial stress for plants like pathogens and insects, which lead to increased the concentration of anthocyanins via a defense mechanisms in plants (Hargreaves et al., 2008). Similarly, the results of a study carried out by Hemashenpagam and Selvaraj (2011) demonstrated that mycorrhization may influence the metabolic pathway of anthocyanin in plants by activating the biosynthesis of anthocyanin, thereby influencing the accumulation of anthocyanin.
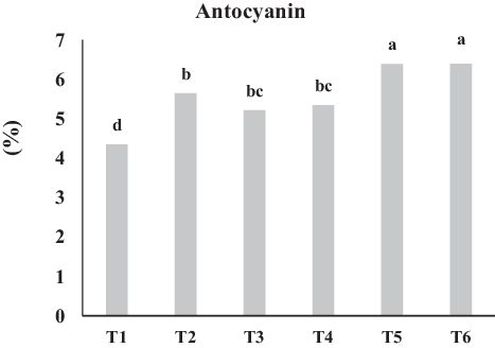
Fig. 3. Effect of bio fertilizers on leaf anthocyanin content. Different letters indicate statistical differences between groups according to DMRT at P ≤ 0.05. T1: Control, T2: MY, T3: RHC, T4: RHB, T5: RHC + MY and T6; RHB + MY.
Flavonoid content was significantly influenced by organic fertilizers application into the soil (P ≤ 0.05). The lowest (0.51%) and the highest (0.71%) concentrations of leaf flavonoid content was measured in the control and combined treatment of RHB + MY respectively. Combined application of RHC and RHB along with MY were more effective in comparison with separate application and leaf flavonoid concentrations were increased by 37.96% and 39.92% compared to control, respectively (Fig. 4). As bio-fertilizer application can improve soil physicochemical and biological conditions, secondary metabolite concentrations can be affected via an enhanced water and nutrient uptake rate of plants. There was a positive correlation between nutrient uptake, especially N uptake, and flavonoid content (Table 3). These improvements in crop performance are consistent with other studies describing flavonoids as practical indicators of both potential photosynthetic productivity and plant vigor. These are related to the N concentration and serve as a response of crop to N fertilizer application and soil nutrient status (Bozzolo et al., 2017). This clearly demonstrates that flavonoid content is influenced by bio-fertilizer. (Veberic et al., 2005). Similarly, the results of a study showed that application of organic fertilizer (10% N: 10% P2O5: 10% K2O) increased the production of flavonoids in Pumila Benth. (Ibrahim et al., 2013). The examination carried out by Tabaldi et al. (2016) described that, flavonoid contents were significantly affected by addition of poultry manure in the cultivation of Pink Pepper, which was traced back to the high concentration of nitrogen in poultry manure (Jones and Hartely, 1999). In another research the total flavonoid content increased by 26% in leaves of Manihot esculenta in response to vermicompost compared to inorganics fertilizer sources (Nur et al., 2013). Based on the other researchers` results, it was concluded that the concentration of flavonoids increased in all of the inoculated plants with mycorrhiza. In fact, flavonoids play a significant role in how plants interact with organisms and serve as signaling molecules between plants and soil microorganisms. (Francineyde et al., 2014; Hemashenpagam and Selvaraj, 2011). Similarly to our results, it was documented that colonization with mycorrhizal fungi influenced total flavonoid content in plants (Mollavi et al., 2015), which might be due to the efficacy of mycorrhization on production of flavonoids during symbiosis and also by changing the expression of genes involved in biosynthesis of flavonoids (Devi and Reddy, 2002). The results of a study that investigated the impact of mycorrhization on flavonoid concentration demonstrated a significant increase in flavonoid content in Viola tricolor L., which may be because of an improvement in nutrient status induced by mycorrhiza or due to the plant’s systemic defense reaction to inoculation (Zubek et al., 2015). According to what was reported by Zhang et al. (2013), improved formation of flavonoids caused by mycorrhizal inoculation in Ferrea, can be explained by the activity of key-enzymes responsible for flavonoid synthesis. Accumulation of plant secondary metabolites like flavonoids in response to mycorrhization may be attributed to the enhancement in mechanisms such as activation of metabolic routes, production of signaling molecules and alterations in the activity of key-enzymes for the production of these compounds (Riter Netto et al., 2014). Furthermore the increased level of total flavonoids in plants as a results of mycorrhization can be due to the reaction of the plant against mycorrhizal colonization induced by phenolic compound accumulation in plant cells as a plant defense response against microorganisms (Lu et al., 2015).
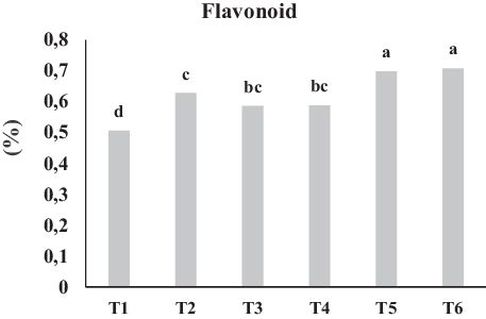
Fig. 4. Effect of bio fertilizers on plant flavonoid content. Different letters indicate statistical differences between groups according to DMRT at P ≤ 0.05. T1: Control, T2: MY, T3: RHC, T4: RHB, T5: RHC + MY and T6; RHB + MY.
Accumulation of mucilage was significantly (p ≤ 0.01) affected by the studied treatments. Results revealed that combined application of bio-fertilizers was more effective than separate application. The lowest (8.0%) and the highest (11.1%) accumulation of mucilage in plant leaves were related to the control and combination treatment of RHB + MY, respectively. All treatments significantly influenced mucilage content in plant leaves ranging from 12.3% to 39.1% in comparison with the control (Fig. 5). Regarding to the previous results of this experiment and with respect to the effect of bio-fertilizers application, it can be stated that application of bio-fertilizers improved plant nutrient availability which affects secondary metabolite formation. The positive correlation between nutrient uptake, chlorophyll pigments, and mucilage accumulation in plants indicated an effective influence of organic matter application on plant growth and secondary metabolite quantities (Table 3). The most important role of mucilage is to facilitate freezing tolerance and protect plant against water stress via absorbing water from the atmosphere and storing it, hence, the amount of mucilage is considered as a plant healthy factor (Patten et al., 2010). In conformity to our findings, there are numerous studies reporting that mucilage accumulation, as a production of many various plant species will be affected by organic matter application (Raei and Alami-Milani, 2014; Ebrahimi et al., 2010). Moreover, according to the results of an experiment, a significant difference was observed in terms of mucilage concentration between treatments in which the highest mucilage content belonged to the organic fertilizer application (19%) and the lowest content was related to the control (8%) (Mirshekari and Forouzandeh, 2015). Generally, the increased percentage of mucilage followed by organic matter application may be due to different factors such as increased absorption content of nutrients in plant (Bajiya, 1994). On the other hand, a positive effect of mycorrhiza on mucilage percentage in medicinal plants has been reported, which refers to enhanced nutritional status in mycorrhizal plants. (Raei and Weria, 2013). Significant increase in mucilage content following bio-fertilizers application and mycorrhization is in agreement with other studies describing that enhancement in nutrients availability induced by bio-fertilizer addition significantly increased mucilage content. (Hendawy, 2008; Abedini et al., 2015; Khalid and Khalid, 2012).

Fig. 5. Effect of bio fertilizers on plant mucilage accumulation. Different letters indicate statistical differences between groups according to DMRT at P ≤ 0.05. T1: Control, T2: MY, T3: RHC, T4: RHB, T5: RHC + MY and T6; RHB + MY.
Application of bio-fertilizers significantly increased total carbohydrate content compared to the control (P ≤ 0.01). Combined application of bio-fertilizers was more effective than separate application and showed that the highest concentrations of carbohydrate which were 12.3 and 12.0 mg g–1 fw, was measured in the RHB + My and RHC + MY treatments, respectively. The lowest content of total carbohydrate was measured in the control, which was 8 mg g–1 fw (Fig. 6). Our results are in agreement with those of researchers who reported that compost application at different rates improved carbohydrate content (Aminifard et al., 2013; Winter and Davis, 2006). Moreover, it was concluded that carbohydrate percentage increased by using compost, which may be attributed to the positive effect of compost as a source of essential nutrients besides improving their availability (Mazhar et al., 2011). Based on the data presented in Table 3, it can be concluded that there is a positive correlation between chlorophyll pigments and total carbohydrate concentration in plants. El-Quesni et al. (2013) observed a significantly increased total carbohydrates content in leaves of Jatropha followed by bio-fertilizer application, which was due to efficiency of photosynthesis of the plants. In general, the enhanced carbohydrate status of leaves might be due to an increased in chlorophyll content and photosynthetic rate coupled with enhanced synthesis of carbohydrate (Elham and Rania, 2015). Following compost application, Abd El-Sabour et al. (1997) reported an improvement in plant efficiency for carbohydrate production via an increase in total chlorophyll formation. In agreement with our findings, there are numerous studies describing the effectiveness of mycorrhization on total carbohydrate content (Raei and Weria, 2013). According to the results of an experiment it was indicated that the measured amount of total carbohydrate in mycorrhizal plants was higher in comparison with non-mycorrhizal plants (Shinde and Manjusha, 2014),which could be due to the mechanisms causing rapid uptake and movement of carbohydrate from fungus to host (Lenin et al., 2010). Furthermore the positive correlation between mycorrhizal association and carbohydrates content in plant can be due to the enhancement of nutrient availability for plants, which positively affect plant secondary metabolite synthesis such as chlorophyll, hormones, vitamins and carbohydrate (Ocampo and Azcon, 1985). Additionally Wu et al. (2011) illustrated that mycorrhization increased plant photosynthetic rate relative to non-mycorrhizal plants, which may have led to higher carbohydrate concentrations in mycorrhizal plants.
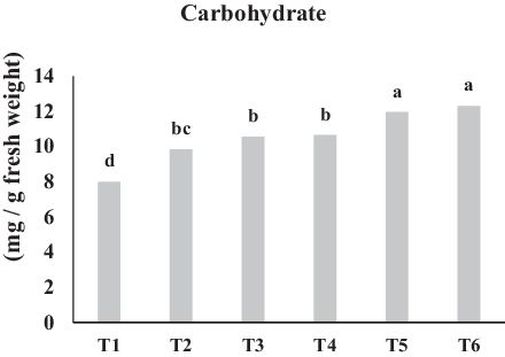
Fig. 6. Effect of bio fertilizers on total carbohydrate content. Different letters indicate statistical differences between groups according to DMRT at P ≤ 0.05. T1: Control, T2: MY, T3: RHC, T4: RHB, T5: RHC + MY and T6; RHB + MY.
The findings of the present research revealed that all studied treatments in Echium cultivation significantly affected the amount of leaf dry matter yield, chlorophyll pigment content, proline, anthocyanin, flavonoid, mucilage and carbohydrate concentrations. Bio-fertilizer application significantly influenced the studied parameters via an increase in the levels of NPK and an enhancement in nutrient availability in soil. An important result of this research was the considerable effect of enriched RHC and RHB with mycorrhiza on the studied traits. Combined application of bio-fertilizers with mycorrhiza was superior to separate applications in increasing leaf dry matter yield and all measured parameters. The role of mycorrhization on nutrient uptake rate by plants via creating an elaborate web of hyphae that confines and absorbs nutrients was significant compared to the control. Enhancement of nutrient availability for plants positively affected plant secondary metabolite synthesis. Furthermore mycorrhization may influence the metabolic pathway of anthocyanin in plants and activates the key-enzymes, which are responsible for biosynthesis of secondary metabolite. Our results suggest that RHC, RHB application in combination with MY application improved plant nutrient uptake rate and enhanced growth/yield and plant secondary metabolites status. Further research is needed to determine long-term impacts of these soil conditioners on the secondary metabolite status of medicinal plants.
Abd El-Sabour, M.F., M.A. Abo El-Seoud, M. Rizk, 1997: Physiological and chemical responses of sunflower to the application of previous organic waste composts to sandy soil. Environmental Management and Health 8 (4), 128-132.
Abdullah, A.T., M.S. Hanafy, E.O. El-ghawwas, Z.H. Ali, 2012: Effect of compost and some biofertilizers on growth, yield, essential oil productivity and chemical composition of Rosmarinus officinalis, L. plants. Journal of Horticultural Science and Ornamental Plants 4 (2), 201-214, DOI: 10.5829/idosi.jhsop.2012.4.2.248.
Abedini, T., P. Moradi, A. Hani, 2015: Effect of organic fertilizer and foliar application of humic acid on some quantitative and qualitative yield of Pot marigold. Journal of Novel Applied Sciences 4 (10), 1100-1103.
Agatonovic-Kustrin, S., D. Babazadeh Ortakand, D.W. Morton, A.P. Yusof, 2015: Rapid evaluation andcomparison of natural products and antioxidant activity in calendula, feverfew, and German chamomile extracts. Journal of Chromatography A 13 (1385), 103-10, DOI: 10.1016/j.chroma.
Agegnehu, G., A.M. Bass, P.N. Nelson, M.I. Bird, 2016: Benefits of biochar, compost and biochar–compost for soil quality, maize yield and greenhouse gas emissions in a tropical agricultural soil. Science of the Total Environment 543, 295-306.
Agegnehu, G., M.I. Bird, P.N. Nelson, A.M. Bass, 2015: The ameliorating effects of biochar and compost on soil quality and plant growth on a Ferralsol. Soil Research 53 (1), 1–12, DOI: 10.1071/SR14118.
Allah Wadhayo, G., B. Shah faisal, S. Mohammad saleem, G. Rabail, L. Muhammad Siddique, 2015: Impact of rice husk biochar and macronutrient fertilizer on fodder maize and soil properties. International Journal of Biosciences 7 (4), 12-21.
Amei, L., T. Daike, X. Yanci, M. Haibo, 2016: Biochar improved growth of an important medicinal plant (Salvia miltiorrhiza Bunge) and inhibited its cadmium uptake. Journal of Plant Biology and Soil Health (2), 6.
Aminifard, M., H. Aroiee, M. Azizi, H. Nemati, J. Hawa, 2013: Effect of compost on antioxidant componentsa nd fruit quality of sweet pepper (capsicum annuum L.). Journal of Central European Agriculture 14 (2), 47-56, DOI: /10.5513/JCEA01/14.2.1232.
Bajiya, H.S, 1994: Response of fenugreek to phosphorous and sulfur. M.Sc. Thesis, Rajasthan Agricultural university, Bikaner.
Bates, L.S., R.P. Waldren, I.D. Tear, 1973: Rapid determination of free proline for water-stress studies. Plant and soil 39 (1), 205-207, DOI: 10.1007/BF00018060.
Bozzolo, A., D. Pizzeghello, A. Cardinali, O. Francioso, S. Nardi, 2017: Effects of moderate and high rates of biochar and compost on grapevine growth in a greenhouse experiment. AIMS Agriculture and Food 2 (1), 113-128, DOI: 10.3934/agrfood.2017.1.113.
Bremner, J.M, 1996: Nitrogen total. In: Methods of soil analysis, part 3. Chemical Methods. D. L. SPARKS et al. (Ed.), 085–1122. 3rd ed. Madison, WI: American Society of Agronomy, Inc.
Briccoli, B.C., E. Santilli, L. Lombardo, 2015: Effect of arbuscular mycorrhizal fungi on growth and on micronutrient and macronutrient uptake and allocation in olive plantlets growing under high total Mn levels. Mycorrhiza 25 (2), 97-108, DOI: 10.1007/s00572-014-0589-0.
Cabello, M., G. Irrazabal, A.M. Bucsinszky, M. Saparrat, S. Schalamuk, 2005: Effect of an arbuscular mycorrhizal fungus, Glomus mosseae, and a rock-phosphate-solubilizing fungus, Penicillium thomii, on Mentha piperita growth in a soilless medium. Journal of Basic Microbiology 45 (3), 182-189, DOI: 10.1002/jobm.200410409.
Cercioglu, M., B. Okur, S. Delibacak, A.R. Ongun, 2014: Changes in physical conditions of a coarse textured soil by addition of organic wastes. Eurasian Journal of Soil Science 3 (1), 7-12. Doi: 10.18393/ejss.47968.
Chang, C.C., M.H. Yang, H.M. Wen, J.C. Chern, 2002: Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food and Drug analysis 10 (3), 178-182.
Chapman, H.D., P.F. Pratt, 1961: In Methods of analysis for soils, plants and waters. Berkeley, California, USA: The University of California’s Division of Agricultural Science.
Conversa, G., A. Bonasia, C. Lazzizera, A. Elia, 2015: Influence of biochar, mycorrhizal inoculation, and fertilizer rate on growth and flowering of Pelargonium (Pelargonium zonale L.) plants. Original Research, DOI: 10.3389/fpls.2015.00429.
Devi, M.C., M.N. Reddy, 2004: Effect of arbuscular mycrrhizal fungi and rhizobium association on chlorophyll content of ground nut (Arachis hypogaea L.) Mycorrhiza news 6 (1), 15-17.
Devi, M.C., M.N. Reddy, 2002: Phenolic acid metabolism of groundnut (Arachis hypogaea L.) plants inoculated with VAM fungus and rhizobium. Journal of Plant Growth Regulation 37 (2), 151-156.
Dorais, M., D.L. Ehre, A.P. Papadopoulos, 2008: Tomato (Solanum lycopersicum) health components: from the seed to the consumer. Phytochemistry Reviews 7, 231-250.
Dubois, M., K.A. Gilles, J.K. Hamilton, P.A. Rebers, F. Smith, 1956: Colometric method for determination of sugar and related substances. Analytical Chemistry 28, 350-356.
Ebrahimi, A., P. Moaveni, H. Aliabadi farahani, 2010: Effects of planting dates and compost on mucilage variations in borage (Borago officinalis L.) under different chemical fertilization systems. International Journal for Biotechnology and Molecular Biology Research 1 (5), 58-61.
Egamberdieva, D., S. Wirth, U. Behrendt, E.F. Abd allah, G. Berg, 2016: Biochar treatment resulted in a combined effect on soybean growth promotion and a shift in plant growth promoting rhizobacteria. Frontiers in Microbiology 25 (7), 209, DOI: 10.3389/fmicb.2016.00209.
Elham, M.A., M.K. Rania, 2015: Effect of different planting dates and organic fertilizers treatments on growth and yield of Hibiscus Sabaariffa L. plants. Egyptian Journal of Desert Research 65 (1), 153-170.
El-Quesni, F.E.M., Kh. I. Hashish, m. M kandil, A.A.M. Mazher, 2013: Impact of some bio-fertilizers and compost on growth and chemical composition of Jatropha curcas. World Applied Sciences Journal 21 (6), 927-932, DOI: 10.5829/idosi.wasj.2013.21.6.2907.
El-Quesni, F.E.M., S.M. Zaghloul, S.S. Hanan, 2010: Effect of microbien and compost on growth and chemical composition of Schefflera arboricola L. under salt stress. Journal of American Science 6 (10), 1073-1080.
Enami, I., A. Okumura, R. Nagao, 2008: Structures and functions of the extrinsic proteins of photosystem II from different species. Photosynthesis Research 98 (1-3), 349-363, DOI: 10.1007/s11120-008-9343-9.
Feng, W., L. He, H.Y. Zhang, B.B. Guo, Y.J. Zhu, C.Y. Wang, T.C. Gua, 2015: Assessment of plant nitrogen status using chlorophyll fluorescence parameters of the upper leaves in winter wheat. European Journal of Agronomy 64, 78-87, DOI.org/10.1016/j.eja.2014.12.013.
Follet, R., R. Donahue, L. Murphy, 1981: Soil and soil amendments. Prentice-Hall, Inc., New Jersey.
Francineyde, A.S., S.B.S. Fabio, C.M. Leonor, 2014: Biotechnical application of arbuscular mycorrhizal fungi used in the production of foliar biomolecules in ironwood seedlings Libidibia ferrea (Mart. ex Tul.) L. P. Queiroz var. ferrea. Journal of Medicinal Plant Research 8 (20), 814-819, DOI: 10.5897/JMPR2014.5358.
Gillman, G.P., E.A. Sumpter, 1986: Modification to the compulsive exchange method for measuring exchange characteristics of soils. Australian Journal Soil Researcher 24 (1), 61-66, DOI: 10.1071/SR9860061.
Hargreaves, J.C., M.S. Adl, P.R. Warman, H.P.V. Rupasinghe, 2008: The effects of organic and conventional nutrient amendments on strawberry cultivation: Fruit yield and quality. Journal of the Science of Food and Agriculture 88 (15), 2669-2675.
Heitkotter, J., B. Marschner, 2015: Interactive effects of biochar ageing in soils related to feedstock, pyrolysis temperature, and historic charcoal production. Geoderma 245, 56-64, DOI: 10.1016/j.geoderma.2015.01.012.
Hemashenpagam, N., T. Selvaraj, 2011: Effect of arbuscular mycorrhizal (AM) fungus and plant growth promoting rhizomicroorganisms (PGPR’s) on medicinal plant Solanum viarum seedlings. Journal of Environment Biology 32 (5), 579-583.
Hendawy, S., A. Khalid, 2011: Effect of chemical and organic fertilizers on yield and essential oil of chamomile flower heads. Medicinal and Aromatic plant science and biology 5(1), 43- 48.
Hendawy, S.F, 2008: Comparative study of organic and mineral fertilization on Plantago arenaria plant. Journal of Applied Sciences Research 4 (5), 500-506.
Higo, M., K. Isobe, D.J. Kang, K. Ujie, R.A. Drijber, R. Ishii, 2010: Inoculation with arbuscular mycorrhizal fungi or crop rotation with mycorrhizal plants improves the growth of maize in limed acid sulfate soil. Plant Production Science 13(1),74-79.
Hirst, E.L., J.K.N. Jones, 1955: The Analysis of Plant Gums and Mucilages: Part of the Modern Methods of Plant Analysis. In H. F. Linskens, K. Paech, B. D. Sanwal and M. V. Tracy (Eds), Pflanzenanal, volume 2 Springer (pp. 275-294).
Hosseni vakili, R., S. Ghanbari, 2015: Comparative examination of the effect of manure and chemical fertilizers on yield and yield components of rosemary (Rosemarinus officinalis L.). International Journal of Agronomy and Agricultural Research 6 (2), 29-37.
Hosseini Vakili, R., S. Ghanbari, M. Akbarzadeh, M. Ghasempour, alamdari, S. Golmohammadzadeh, 2015: Effect of organic and chemical fertilizers on dry yield, essential oil and compounds on rosemary (Rosemarinus officinalis L.). Biological Forum – An International Journal 7 (1), 773-782.
Ibrahim, M.H., Z.E.J. Hawa, E. Karimi, A. Ghasemzadeh, 2013: Impact of organic and inorganic fertilizers application on the phytochemical and antioxidant activity of Kacip Fatimah (Labisia pumila Benth). Molecules 18 (9), 10973-10988, DOI: 10.3390/molecules180910973.
Jones, C.G., S.E.A. Hartely, 1999: Protein competition model of phenolic allocation. Journal article, Oikos, Rio de Janeiro 86 (1), 27-44.
Kannayan, S., 2002: Biofertilizer for sustainable crop production: Biotechnology of biofertilizers. In S. KANNAYAN (Ed), Narosa Publishing House (pp. 9-49). New Delhi, India.
Karanatsidis, G., M. Berova, 2009: Effect of organic-N fertilizer on growth and some physiological parameters in Pepper plants (Capsicum Annum L.). Journal of Biotechnology & Biotechnological Equipment 23 (1), 254-257.
Karlen, D.L., M.J. Mausbach, J.W. Doran, R.G. Cline, R.F. Harrise, G.E. Schuman, 1997: Soil quality: A concept, definition and framework for evaluation. Soil Science Society of American Journal 61 (1), 4-10, DOI: 10.2136/sssaj1997.03615995006100010001x.
Karthikeyan, b., m. M. Joe, c. A. Jaleel, 2009: Response of some medicinal plants to vesicular arbuscular mycorrhizal inoculations. Journal of scientific research 1 (1), 381-189.
Khalid, A., A. Khalid, 2012: Biological fertilization and its effect on medicinal and aromatic plants. Nusantar a Bioscience 4 (3), 124-133.
Kumar, A., C. Mangla, A. Aggarwal, 2015: Significant effect of mycorrhization on some physiological parameters of Salvia officinalis Linn. plant. International Journal of Current Microbiology and Applied Sciences 4 (5), 90-96.
Lehmann, J., J. Gaunt, M. Rondon, 2006: Bio-char sequestration in terrestrial ecosystems. A review. Mitigation and Adaptation Strategies for Global Change 11 (2), 403-427, DOI: 10.1007/s11027-005-9006-5.
Lenin, M., G. Selvakumar, P. Thamizhiniyan, R. Rajendiran, 2010: Growth and biochemical changes of vegetable seedlings induced by arbuscular mycorrhizal fungus. Journal of Experimental Sciences 1 (4), 27-31.
Liang, C., G. Gasco, S. Fu, A. Mendez, J. Paz-ferreiro, 2016: Biochar from pruning residues as a soil amendment: effects of pyrolysis temperature and particle size. Soil and Tillage Research 164, 3-10, DOI: 10.1016/j.still.2015.10.002.
Lingua, G., E, Bona, P. Manassero, F. Marsano, V. Todeshini, S. Cantamessa, A. Copetta, G. D’agostino, E. Gamalero, G. Berta, 2013: Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads increases anthocyanin concentration in strawberry fruits (Fragaria × ananassa var. Selva) in conditions of reduced fertilization. International Journal of Molecular Sciences 14 (8), 16207-16225, DOI: 10.3390/ijms140816207.
Lu, F.C., C.Y. Lee, C.L. Wang, 2015: The influence of arbuscular mycorrhizal fungi inoculation on yam (Dioscorea spp.) tuber weights and secondary metabolite content. PeerJ 3: e1266, DOI: 10.7717/peerj.1266.
Maie Mohsen, M., A. Hanna, A. Abo-kora, H.M. Abeer kassem, 2016: Effect of vermicompost and calcium silicate to reduce the soil salinity on srowth and oil determinations of marjoram plant. International Journal of ChemTech Research 9 (5), 235-262.
Markus, A.S., R. Syed omar, J. Shamshuddin, C.I. Fauziah, 2008: Changes in properties of composting rice husk and their effects on soil and cocoa growth. Communications in Soil Science and Plant Analysis 39 (15-16), 2221-2249, DOI: 10.1080/00103620802289117.
Mazhar, A.A.M., N.G. Abdel-aziz, I. Sh. Shaymaa, S.M. Zaghloul, 2011: Effect of nile compost application on growth and chemical constituents of Jatropha curcas grown under different salinity levels of diluted sea water. Australian Journal of Basic and Applied Sciences 5 (9), 967-974.
Mehrbani, M., N. Ghasemi, E. Sajjadi, A. Ghannadi, M. Shams-ardakani, 2005: Main compounds of petals of Echium amoenum Fisch. and C.A. Mey., a famous medicinal plant of Iran. DARU 13(2), 65-69.
Miraj, S., S, kiani, 2016: A review study of therapeutic effects of Iranian borage (Echium amoenum Fisch). Scholars Research Library 8 (6), 102-109.
Mirshekari, S., M. Forouzandeh, 2015: Analysis of effect of organic fertilizer on yield and active substance of psyllium herbal plant (Plantago ovata L.). Biological Forum – An International Journal 7 (1), 436-440.
Mollavi, M., S. Bolandnazar, H. Nazemieh, N. Aliasgharzad, 2015: The effect of mycorrhizal fungi on antioxidant activity of various cultivars of onion (Allium cepa L). International Journal of Biosciences 6 (1), 66-79, DOI: 10.12692/ijb/6.1.66-79.
Morteza-Semnani, K., M. Saeedi, 2013: Essential oil composition of Echium amoenum Fisch. & C.A. Mey. Journal of Essential Oil Bearing Plants 8 (1), 61-64, DOI: 10.1080/0972060X.2005.10643422.
Nanjo, T., M. Fujita, M. Seki, T. Kato, S. Tabata, K. Shinozaki, 2003:Toxicity of free proline revealed in an arabidopsis T-DNA-tagged mutant deficient in proline dehydrogenase. Plant and Cell Physiology 44 (5), 541-8.
Neumann, E., E. George, J. Emir, 2009: The effect of arbuscular mycorrhizal root colonization on growth and nutrient uptake of two different cowpea (Vigna unguiculata L. Walp.) genotypes exposed to drought stress. Journal of the Science of Food and Agriculture 21 (2), 01-17, DOI: 10.9755/ejfa.v21i2.5160.
Nur, F.O., A.H. Siti, K.Y. Umi, 2013: Comparative evaluation of organic and inorganic fertilizers on total phenolic, total flavonoid, antioxidant activity and cyanogenic glycosides in cassava (Manihot esculenta). African Journal of Biotechnology 12(18), 2414-2421.
Ocampo, J.A., R. Azcon, 1985: Relationship between the concentration of sugars in the roots and VA mycorrhizal infection. Plant and Soil 86 (1), 95-100.
Patten, A.M., D.G. Vassao, M.P. Wolcott, L.B. Davin, N.G. Lewis, 2010: Trees: a remarkable biochemical bounty. Comprehensive natural products II. Chemistry and biology. L. MANDER, L. H. WEN (Ed.), 1st edition, Elsevier Science Published (pp. 1173-12963).
Porra, R., J. Thompson, W.A.P.E. Kriedmann, 1989: Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochimica et Biophysica Acta 975, 384-394.
Raei, Y., M. Alami-Milani, 2014: Organic cultivation of medicinal plants. Journal of Biodiversity and Environmental Sciences 4 (4), 6-18.
Raei, Y., W. Weria, 2013: Arbuscular mycorrhizal fungi associated with some aromatic and medicinal plants. Bulletin of Environment, Pharmacology and Life Sciences 2 (11), 129-138.
Rajkovich, S., A. Enders, K. Hanley, C. Hyland, A.R. Zimmerman, 2012: Corn growth and nitrogen nutrition after addition of biochars with varying properties to a temperate soil. Biology and Fertility of Soils, Cooperating Journal of International Society of Soil Science 48 (3), 271-284, DOI: 10.1007/s00374-011-0624-7.
Riter netto, A.F., M.S. Freitas, M.A. Martins, A.J. Carvalho, J.A. Vitorazi filh, 2014: Effect of arbuscular mycorrhizal fungi on the bioproduction of total phenols and the growth of Passiflora alata Curtis. Brazilian Journal of Medicinal Plants 16 (1), 1-9, DOI: org/10.1590/S1516-05722014000100001.
Ruscitti, M., M. Arango, M. Ronco, J. Beltrano, 2011: Inoculation with mycorrhizal fungi modifies proline metabolism and increases chromium tolerance in pepper plants (Capsicum annuum L.). Brazilian Journal of Plant Physiology 23 (1), 15-25, DOI: org/10.1590/S1677-04202011000100004.
Salehi, A., H. Tasdighi, M. Gholamhosseini, 2016: Evaluation of proline, chlorophyll, soluble sugar content and uptake of nutrients in the German chamomile (Matricaria chamomilla L.) under drought stress and organic fertilizer treatments. Asian Pacific Journal of Tropical Biomedicine 6 (10), 886–891, DOI: org/10.1016/j.apjtb.2016.08.009.
Santos-Buelga, C., N. Mateus, V. De freitas, 2014: Anthocyanins, plant pigments and beyond. Journal of Agricultural and Food Chemistry 62(29), 6879-84, DOI: 10.1021/jf501950 s.
Selvakumar, G., P. Thamizhiniyan, 2011: The effect of the arbuscular mycorrhizal (AM) fungus glomus intraradices on the growth and yield of Chilli (Capsicum annuum L.) under salinity stress. World Applied Sciences Journal 14 (8), 1209-1214.
Shaalan, M.N, 2005: Effect of compost and different sources of biofertilizers on borage plants (Borage officinalis L.). Egyptian Journal of Agricultural Research 83 (1), 271- 284.
Shamsul, H., H. Qaiser, M.N. Alyemeni, A.S. Wani, J. Pichtel, A. Ahmad, 2012: Role of proline under changing environments. Plant Signaling and Behavior 7 (11): 1456-1466, DOI: 10.4161/psb.21949.
Shinde, B.P., K. Manjusha, 2014: Impact of AM fungi on biochemical changes in potato plants. International Journal of Current Microbiology and Applied Sciences 3 (7), 1018-1027.
Subramanium, T., G. Pandurangan, K. Ramasamy, A. Rangasamy, P. Diby, 2018: Exploration of rice husk compost as an alternate organic manure to enhance the productivity of blackgram in typic haplustalf and typic rhodustalf. International Journal of Environmental Research and public health 15 (2), 358, DOI: 10.3390/ijerph15020358.
Swamy, M.K., M.S. Akhtar, U.R. Sinniah, 2016: Response of PGPR and AM fungi toward growth and secondary metabolite production in medicinal and aromatic plants: plant, soil and microbes. In: K. R. HAKEEM, M. S. AKHTAR (Eds, Springer International Publishing Switzerland (pp. 145-168), DOI: 10.1007/978-3-319-29573-2_7.
Tabaldi, L.A., M.C. Vieira, N.A.H. Zarate, A.S.N. Formagio, M. Pilecco, L.R. Silva, K.P. Santos, L.A.C. Santos, C.A.L. Cardoso, 2016: Biomass yield and flavonoid and phenol content of Schinus terebinthifolius cultivated in single or double row with poultry litter. Ciencia Florestal 26 (3), 787-796, DOI: org/10.5902/1980509824207.
Tag, A.T., G. Duman, S. Ucar, J. Yanik, 2016: Effect of feedstock type and pyrolysis temperature on potential applications of biochar. Journal of Analytical and Applied Pyrolysis 120, 200-206, DOI: 10.1016/j.jaap.2016.05.006.
Veberic, R., M. Trobec, K. Herbinger, M. Hofer, D. Grill, F. Stampar, 2005: Phenolic compounds in some apple (Malus domestica Borkh.) cultivars of organic and integrated production. Journal of the Science of Food and Agriculture 85 (10), 1687-1694, DOI: org/10.1002/jsfa.2113.
Wanger, G.J, 1979: Content and vacuole/extravacuole distribution of neutral sugars, free amino acids, andanthocyanin in protoplasts. Plant Physilogy 64 (1), 88-93.
Winter, C.K., S.F. Davis, 2006: Organic foods. Journal of Food Science 71 (9), 117-124, DOI: 10.1111/j.1750-3841.2006.00196.x.
Wu, J., B. Sun, Y. Wang, G. Xin, S. Ye, S. Peng, 2011: Arbuscular mycorrhizal fungal colonization improves regrowth of Bermudagrass (Cynodon Dactylon L.) after cutting. Pakistan Journal of Botany 43 (1), 85-93, DOI: 10.1090/mbk/079/06.
Xue, X., A. Liu, X. Hua, 2009: Proline accumulation andtranscriptional regulation of proline biosynthesis and degradation in Brassica napus. US National Library of Medicine National Institutes of Health 31 (42), 28- 34.
Yadav, R.L., G.L. Keshwa, S.S. Yadwa, 2003: Effect of integrated use of FYM and sulphure on growth and yield of isabgol. Journal of Medicinal and Aromatic Plant Sciences 25, 668-671.
Yamuangmorn, S., B. Dell, B. Rerkasem, C. Prom-u-thai, 2018: Applying nitrogen fertilizer increased anthocyanin in vegetative shoots but not in grain of purple rice genotypes. Journal of the Science of Food and Agriculture, First published, DOI: org/10.1002/jsfa.8978.
Yang, C.W., C.C. Lin, C.H. Kao, 2000: Proline, ornithine, arginine and glutamic acid contents in detached rice leaves. Biologia Plantarum 43 (2), 305-307.
Zahed Chkovari, S., S. Enteshari, N. Qasimov, 2015: Effect of salinity stress on biochemical parameters and growth of borage (Borago officinalis L.). Iranian Journal of Plant Physiology 6 (2), 1673-1685.
Zhang, R.Q., H.H. Zhu, H.Q. Zhao, Q. Yao, 2013: Arbuscular mycorrhizal fungal inoculation increases phenolic synthesis in clover roots via hydrogen peroxide, salicylic acid and nitric oxide signaling pathways. Journal of Plant Physiology 170 (1), 74-79, DOI: org/10.1016/j.jplph.2012.08.022.
Zhang, D., G. Pan, G. Wu, G.W. Kibue, L, li, X. Zhang, J. Zheng, K. Cheng, S. Joseph, 2016: Biochar helps enhance maize productivity and reduce greenhouse gas emissions under balanced fertilization in a rainfed low fertility inceptisol. Chemosphere 142, 106-113, DOI: 10.1016/j.chemosphere.2015.04.088.
Zolfaghari, M., V. Nazeri, F. Sefidkon, F. Rejali, 2013: Effects of arbuscular mycorrhizal fungi on plant growth and essential oil content and composition of Ocimum basilicum L. Iranian Journal of Plant Physiology and Biochemistry 3 (2), 643-650.
Zubek, S., K. Rola, A. Szewszyk, M.L. Majewska, K. Turnau, 2015: Enhanced concentrations of elements and secondary metabolites in Viola tricolor L. induced by arbuscular mycorrhizal fungi. Plant and Soil 390 (1-2), 129-142.
Zuccarini, P, 2007: Mycorrhizal infection ameliorates chlorophyll content and nutrient uptake of lettuce exposed to saline irrigation. Plant Soil and Environment 53 (7), 283-289.
Footnotes:
Classification: Fungi, Mucoromyceta, Glomeromycota, Glomeromycotina, Glomeromycetes, Glomerales, Claroideoglomeraceae, Claroideoglomus | |
Revolutions per minute | |
Classification: Fungi, Mucoromyceta, Glomeromycota, Glomeromycotina, Glomeromycetes, Diversisporales, Gigasporaceae, Gigaspora. |