

Assessing the efficacy of bee promoting measures (Hymenoptera, Apiformes) along an urban-rural gradient
Beurteilung der Wirksamkeit von bienenfördernden Maßnahmen (Hymenoptera, Apiformes) entlang eines Stadt-Land-Gradienten
Journal für Kulturpflanzen, 72 (5). S. 173–184, 2020, ISSN 1867-0911, DOI: 10.5073/JfK.2020.05.07, Verlag Eugen Ulmer KG, Stuttgart
 This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/deed.en).
This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/deed.en).Cities are emerging as refugia for pollinators, among which bees play a pivotal role for maintaining ecosystem functioning in agricultural and urban settings. While measures to promote bees have been investigated predominantly in the agricultural or rural context, a wide knowledge gap persists with regard to the effectiveness of such measures within urban landscapes. In order to guide research addressing this lack of knowledge, the aim of this perspective paper is to give an overview of the recent research activities based on the published peer-reviewed literature. While research on flower seed mixtures in general focuses on nutritional aspects, studies on plantings of perennial herbs are relatively limited to few plant taxa. Implementation of comparable case studies investigating the effects of tree plantings on bee populations is hampered by a lack of methodological standardization. The conservation value of providing nesting sites in cities needs to be further investigated, in particular concerning ground-nesting bee species. While several case studies indicate a nutritional supporting function of green roofs for urban bee populations, findings with regard to vertical isolation remain equivocal. Various factors driving bee diversity and population structure in the urban context at the local and landscape scale have been identified, the reported relevant landscape scale being represented by radii between 500 and 1000 m in most cases. Future study designs reflecting a continuous and complete gradient of urbanization will be helpful in comparing results on bee promoting measures in agricultural landscapes (which are numerous) to urban settings (which are still encountered much less frequently). Studies looking into the genetic structure of bee populations with regard to urbanization so far represent only a tiny fraction of bee diversity, and the further development of molecular methods could yield novel tools for assessing the success of bee promoting measures in terms of habitat connectivity in the near future.
Key words: bees, Hymenoptera, conservation, measure, urban, rural, gradient
Die Bedeutung von Städten als Refugien für Bestäuberinsekten zeichnet sich zunehmend ab. Bienen spielen eine wichtige Rolle in der Stabilisierung von Ökosystemen, sowohl im ruralen als auch im urbanen Kontext. Während bienenfördernde Maßnahmen vor allem im landwirtschaftlichen bzw. ruralen Kontext untersucht wurden, besteht eine große Wissenslücke in Bezug auf die Effektivität solcher Maßnahmen in urbanen Landschaften. Ziel dieses Übersichtsartikels ist es, einen Überblick über jüngere Forschungsaktivitäten basierend auf der im Peer-Review-Verfahren publizierten Literatur zu geben, um Empfehlungen für zukünftige Forschungsprojekte zum Schließen dieser Wissenslücke abzuleiten. Während Studien zu Saatgutmischungen hauptsächlich auf Ernährungsaspekte abzielen, sind Studien zu Staudenpflanzungen auf vergleichsweise wenige Pflanzentaxa beschränkt. Die Durchführung vergleichbarer Studien zu Effekten von Baumpflanzungen auf Bienenpopulationen wird durch eine geringgradige Methodenstandardisierung erschwert. Der Naturschutzwert künstlicher Niststrukturen in Städten bedarf weiterer Erforschung, insbesondere im Hinblick auf bodennistende Bienenarten. Während mehrere Fallstudien auf eine Ernährungsfunktion von Gründächern für urbane Bienenpopulationen hindeuten, sind die Ergebnisse bezüglich der vertikalen Isolation von Gründächern nicht eindeutig. Zahlreiche Faktoren wurden identifiziert, die die Diversität und Populationsstruktur im urbanen Raum auf lokaler und Landschaftsebene beeinflussen. Der relevante Landschaftsmaßstab wird in den meisten Fällen durch Radien zwischen 500 und 1000 m repräsentiert. Zukünftige Studien, deren Versuchsaufbau einen kontinuierlichen und vollständigen Urbanisierungsgradienten berücksichtigt, werden von Nutzen sein, um die zahlreichen Ergebnisse zu bienenfördernden Maßnahmen in Agrarlandschaften mit den bislang wenigen Ergebnissen in urbanen Landschaften zu vergleichen. Studien, die die genetische Struktur von Bienenpopulationen im Hinblick auf Urbanisierung berücksichtigen, repräsentieren bislang nur einen sehr kleinen Ausschnitt der Bienenvielfalt. Die Weiterentwicklung molekularbiologischer Methoden könnte in naher Zukunft neuartige Werkzeuge zur Bewertung des Erfolgs bienenfördernder Maßnahmen im Hinblick auf die Habitatkonnektivität bereitstellen.
Stichwörter: Bienen, Hymenoptera, Schutzmaßnahmen, urban, rural, Gradient
Cities emerge as refugia for pollinator diversity (Tommasi et al., 2004; Baldock et al., 2015; Sirohi et al., 2015; Hall et al., 2017; Samuelson et al., 2018; but see Cardoso und Gonçalves, 2018, Razo-León et al., 2018; Collado et al., 2019; Fitch et al., 2019b; Harrison et al., 2019). Among pollinators, bees play a pivotal role and are therefore considered a keystone species group. Maintainaing bee diversity is important for ecosystem functioning, not only in agricultural landscapes, but also in urban settings. Conservation of wild bee diversity in urbanized landscapes supports pollination services (Matteson und Langellotto, 2009; Lowenstein et al., 2015), which are positively related to urbanization at the landscape scale (Theodorou et al., 2016). While measures to promote bees have been investigated predominantly in the agricultural or rural context, a wide knowledge gap persists with regard to the effectiveness of such measures within urban landscapes. This is likely to be a consequence of feasibility: better opportunities to replicate study sites within rural compared to urban landscapes (lower costs, larger pool of suitable sampling sites) have probably restricted experimental case studies to agricultural settings (e.g. Byrne und delBarco-Trillo, 2019), while case studies in cities or along an urbanization gradient have been conducted as natural experiments lacking experimental habitat manipulation, e.g. using allotment/community gardens (Matteson et al., 2008; Ahrné et al., 2009; Vaidya et al., 2018), parks (McFrederick und Lebuhn, 2006; Zajdel et al., 2019), public green spaces and botanical gardens (Banaszak-Cibicka und Żmihorski, 2012; Banaszak-Cibicka et al., 2018a; Banaszak-Cibicka et al., 2018b), churchyards and cemeteries (Bates et al., 2011), urban agricultural sites (Bennett und Lovell, 2019), greenroofs (Tonietto et al., 2011; Hofmann und Renner, 2018; Fournier et al., 2020), ornamental flowerbeds (Gunnarsson und Federsel, 2014), vacant lots and urban farms (Sivakoff et al., 2018), golf courses (Threlfall et al., 2015) and wastelands (Twerd und Banaszak-Cibicka, 2019); but see Blackmore und Goulson (2014), Potter et al. (2019) and Fig. 1 for examples involving experimental manipulation. All types of vegetated urban habitat have a potential of improvement, in the sense of promoting bee populations and diversity, through adapted management or conversion. Bee promoting measures may benefit other pollinator and non-pollinator taxa and, depending on the measure, increase floral diversity within cities. While the social sciences and urban planning play an important role in assessing conservation measures, including bee promoting measures, within cities (e.g. van Heezik et al., 2012; Bellamy et al., 2017; Burr et al., 2018; Turo und Gardiner, 2019), more ecological research is needed to guide urban planners in incorporating the needs of bees into their spatially explicit decision taking processes.

Fig. 1. The Model project „Bee City of Braunschweig“, a case study involving bee promoting measures along an urban-rural gradient
Bees need foraging and nesting habitats. They utilize ecological requisites by commuting between different partial habitats, which have to be situated within the commuting flight ranges (i.e. the distances of flights for nest-provisioning and not dispersal distances). Since landscape friction is an important factor underlying the accessibility of food resources for bees and wasps (Johansson et al., 2018), connectivity between habitat patches has to be taken into account especially with regard to urban areas, where habitats tend to be highly fragmented. Habitat corridors can ameliorate potential negative effects of urban environments on pollinator communities (Senapathi et al., 2017). As cities gain importance as conservation areas for bee populations, the importance of connectivity between rural and urban populations increases for the maintenance of pollination services in agricultural crops. Acknowledging this agroeconomic perspective, the creation of corridors between cities and the surrounding area has been put on the political agenda in Germany (Bundesministerium für Umwelt, Naturschutz, Bau und Reaktorsicherheit, 2017).
The aim of this perspective paper is to give an overview of the recent research activities with regard to bee promoting measures in the urban context and to guide research on this topic in the near future towards successful study designs that help clarify so far unresolved issues. Focusing on temperate climates, we deliberately disregarded studies from the tropics.
In addition to the presented bee promoting measures, adapted management of urban green spaces can develop resource poor areas into bee friendly habitats. Simple measures such as mowing less frequently increases the diversity and abundance of bees foraging in suburban lawns (Lerman et al., 2018).
Creating foraging habitats for bees by seeding flower mixtures has been demonstrated as an effective way to promote wild bee populations in the agricultural landscape (e.g Heard et al., 2007; Haaland et al., 2011; Blaauw und Isaacs, 2014; Feltham et al., 2015; Jönsson et al., 2015; Scheper et al., 2015; Williams et al., 2015; Balzan et al., 2016). Very often, effectiveness of a seed mixture is assessed from a purely botanical perspective, i.e. in terms of species richness and abundance of flowering species without taking effects on bee populations into consideration (e.g. Lane et al., 2019).
The vast number of seed mixtures is probably one reason for the high number of studies on this topic (at least in the agricultural context). In general, plant selection focuses on nutritional aspects (nectar and pollen supply; Table 1). Nectar and pollen quantity of plant species used in commercial seed mixtures varies widely, the tested perennial mixture producing more nectar and pollen than the tested annual mixture (Hicks et al., 2016). Potter et al. (2019) used DNA-metabarcoding of pollen sampled by wild bees to assess the extent to which bees forage in wildflower strips sown in an urban environment and to identify key plant species for bee foraging. In an agricultural setting, Warzecha et al. (2018) identified a fraction of plant species contained within four seed mixtures as key species crucial for the flower visiting bee community. Perennial seed mixtures are to be preferred over annual mixtures, in order to allow for a build up of bee populations over multiple years (but see Carvell et al., 2006; Rundlöf et al., 2018). With regard to collection of leaf material used for nest construction, relevant bee species tend to prefer certain plant species over others (MacIvor, 2016b). However, compared to floral food resources, this aspect has so far received little attention in the development of seed mixtures and other bee promoting measures. The same is true for potential provision of above-ground nesting sites, in case the vegetation is (at least in part) not manipulated (e.g. mowed) over two winter periods (Fig. 2).
Table 1. The seed mixture used in the project “Bee City of Braunschweig” (Fig. 1). The mixture consists of 47 annual and perennial plants. The table shows the total number of bee species (excludingApis mellifera andBombus spp.) collecting pollen from these plant taxa, as well as the number of oligolectic (collecting pollen from one or a few plant families) and narrow oligolectic bee species (collecting pollen from one or a few plant genera) collecting pollen from these plant species (data obtained from Westrich, 2018). Based on these data, more than 200 species could benefit from the plants included in the mixture.
Species | Family | Total number of | Number of narrow | Number of |
Achillea millefolium | Asteraceae | 28 | – | 7 |
Anthemis arvensis | Asteraceae | 6 | – | 4 |
Anthemis tinctoria | Asteraceae | 7 | – | 6 |
Anthriscus sylvestris | Apiaceae | 25 | – | 2 |
Ballota nigra | Lamiaceae | 7 | – | 2 |
Barbarea vulgaris | Brassicaceae | 13 | – | 5 |
Betonica officinalis | Lamiaceae | 5 | – | 3 |
Campanula rotundifolia | Campanulaceae | 28 | 10 | – |
Campanula trachelium | Campanulaceae | 17 | 8 | – |
Cardamine pratensis | Brassicaceae | 19 | – | 2 |
Centaurea cyanus | Asteraceae | 8 | – | – |
Centaurea jacea | Asteraceae | 39 | – | 7 |
Centaurea scabiosa | Asteraceae | 31 | – | 7 |
Cichorium intybus | Asteraceae | 38 | – | 10 |
Crepis biennis | Asteraceae | 20 | – | 7 |
Daucus carota | Apiaceae | 25 | – | 4 |
Echium vulgare | Boraginaceae | 38 | 3 | – |
Heracleum sphondylium | Apiaceae | 31 | – | 3 |
Hippocrepis comosa | Fabaceae | 16 | – | 2 |
Hypericum perforatum | Clusiaceae | 16 | – | – |
Hypochaeris radicata | Asteraceae | 33 | – | 11 |
Isatis tinctoria | Brassicaceae | 18 | – | 1 |
Knautia arvensis | Dipsacaceae | 13 | – | 3 |
Lathyrus pratensis | Fabaceae | 10 | 1 | 4 |
Leontodon autumnalis | Asteraceae | 29 | – | 9 |
Leucanthemum vulgare | Asteraceae | 22 | – | 2 |
Matricaria recutita | Asteraceae | 5 | – | 1 |
Medicago lupulina | Fabaceae | 1 | – | – |
Melilotus albus | Fabaceae | 23 | – | 6 |
Papaver rhoeas | Papaveraceae | 10 | – | – |
Picris hieracioides | Asteraceae | 40 | – | 14 |
Plantago media | Plantaginaceae | 7 | – | – |
Ranunculus acris | Ranunculaceae | 43 | 1 | – |
Ranunculus bulbosus | Ranunculaceae | 20 | 1 | – |
Reseda lutea | Resedaceae | 10 | 1 | – |
Reseda luteola | Resedaceae | 4 | 1 | – |
Salvia pratensis | Lamiaceae | 20 | – | – |
Scabiosa columbaria | Dipsacaceae | 7 | – | 3 |
Sinapis arvensis | Brassicaceae | 66 | – | 6 |
Stachys sylvatica | Lamiaceae | 4 | – | 1 |
Tanacetum vulgare | Asteraceae | 21 | – | 7 |
Teucrium scorodonia | Lamiaceae | 5 | – | – |
Trifolium medium | Fabaceae | 3 | – | 2 |
Trifolium pratense | Fabaceae | 28 | – | 7 |
Verbascum lychnitis | Scrophulariaceae | 2 | – | – |
Verbascum nigrum | Scrophulariaceae | 1 | – | – |

Fig. 2. Fall aspect of an uncut wildflower strip in Friedland (Lower Saxony, Germany). Vegetation remaining uncut over two winter periods can provide above-ground nesting opportunities for wild bees suitable for reproduction (Photo: André Krahner).
Plantings of perennial herbs are a bee-promoting measure much less studied compared to flower seed mixtures, probably due to the much higher costs involved in the former. Economical feasibility might be a reason why plantings of herbs are rarely encountered in the agricultural context, where the limited half life of set-aside patches potentially reduces the financial investments. On the opposite, green spaces in cities are much more persistent over time, and the exposure in public spaces probably justifies higher financial investments in such plantings, which citizens generally regard as environmental enrichment. Moreover, unlike seed mixtures, planters can be used for floral enrichment in areas with a high coverage of impermeable substrate. Compared to seed mixtures, plantings allow for a more flexible and target-oriented design of vegetation structure and configuration, thus enabling plant designers to better incorporate aesthetic needs and functional aspects within the urban context into the development of the urban green infrastructure. Studies delivering taxonomically detailed data on flower visitation of plant taxa suitable for planting focus on certain taxa, such as Geranium spp. (Masierowska et al., 2018) and Lobularia maritima (Simao et al., 2018), or aim at potential differences between native and exotic plant species (e.g. Matteson und Langellotto, 2011; Salisbury et al., 2015). Even small patches with planted herbs can have a positive effect on bee communities in a city (Simao et al., 2018).
Research on tree species and cultivars suitable for bee foraging is pivotal for urban bee conservation, since city planners, facing increasing problems of draught stress in city trees, and shifting towards novel tree taxa, lack solid data necessary to take bee foraging into account in their decisions. Few studies have investigated the effect of floral resources in trees on bee populations. While floral resources close to the ground (i.e. flower seed mixtures and plantings of herbs) are easily accessible and can be studied using long-established methods, floral resources in trees are much more difficult to access and the respective methodology is much less standardised. Classical approaches such as transect walks and observation plots are not applicable, and various trap types such as pan traps and flight interception traps need further methodological development in order to yield results that are comparable over multiple studies.
Depending on the species, trees and other flowering woody plants can be used for augmenting floral resources for bees in urban and suburban landscapes (Mach und Potter, 2018). Hausmann et al. (2016) found 19% of the Berlin bee fauna foraging on city trees, with higher visitation rates to tree flowers by wild bees in surroundings with a higher proportion of green spaces. For common city-dwelling bees species, trees can be an important pollen source (MacIvor et al., 2014). Somme et al. (2016) investigated the suitability of widespread urban trees as resources for pollinating insects by analyzing the amount of nectar production as well as the chemical composition of nectar and pollen. In addition to floral resources, honeydew might be an alternative nutritional resource for wild bees offered by trees, at least for bumblebees (Cameron et al., 2019). Willows are an important nutritional resource for bees early in the year. In this dioecious species, male trees support a greater abundance of bees, and species assemblages differ among willow pedigrees (Tumminello et al., 2018).
In addition to nutritional resources, nesting sites (Fig. 3) have to be taken into consideration in urban bee conservation (Fortel et al., 2016). In cities, bee composition can be biased toward cavity-nesting species, while soil-nesting species may occur less frequently due to soil limitation and/or disturbance (Matteson et al., 2008). A number of case studies indicate a trend toward fewer ground-nesting bee species in urban habitats (Hernandez et al., 2009). This may be due to extensive sealing of the soil within cities. For cavity-nesting species, an urban matrix can provide better nesting opportunities compared to the surrounding countryside in some situations (Cane et al., 2006). Everaars et al. (2011) observed effects of microsite conditions on the occurrence of Osmia bicornis in an urban context. Distribution and density of suitable nesting sites play an important role in enhancing bee populations and connectivity among them in urban settings (López-Uribe et al., 2015). So-called ‘bee hotels’ (i.e. artificial nesting structures for above-ground nesting species) are successfully applied in the fields of environmental education and public outreach. However, their potential as measures for conserving bee species remains to be further elucidated (MacIvor and Packer, 2015).

Fig. 3. Urban nesting habitat for ground-nesting bees. Nesting habitat on urban green in Braunschweig (Lower Saxony, Germany; left), with an aggregation of Andrena vaga (right, female with Salix pollen load) in spring (Photos: André Krahner).
Green roofs represent a special case of herb plantings and/or seeding in the city, since the plant species pool for this purpose is very limited. Nevertheless, city dwelling bees use green roofs as forage habitats, e.g. for collection of Sedum pollen (MacIvor et al., 2015), and probably also as nesting sites, to some extent. Due to the microclimate on flat-topped buildings, thermophilic species are probably overrepresented in these habitats, while limited plant species numbers are likely to result in underrepresentation of pollen specialist bees on green roofs (Hofmann und Renner, 2018). At the local scale, plant and bee community composition of green roofs are correlated (Tonietto et al., 2011). Findings with regard to potential vertical isolation, especially for small bee species, remain equivocal (Hofmann und Renner, 2018). Kratschmer et al. (2018) observed higher wild bee diversity and abundance on green roofs with fine substrates and increasing forage availability, and conclude that areas with fine and deeper substrates would benefit eusocial and ground nesting bees. Proportion of green space in the surrounding area is positively correlated with overall bee abundance and species richness (Tonietto et al., 2011) and species richness of cavity nesting bees and wasps (MacIvor, 2016a), emphasizing the importance of connectivity between green roofs and the surrounding habitat.
Urban pollinator communities are influenced by both local and landscape-level factors (Baldock, 2020). Local factors such as flower density, number of plant species (Bates et al., 2011; Fischer et al., 2016) and sun exposure (Everaars et al., 2011) can be driving the distribution of bee species. In cities, floral diversity locally increases bee species richness (Hennig und Ghazoul, 2012; Hamblin et al., 2018). Increasing local temperatures reduce bee abundance in cities (Hamblin et al., 2018), although higher average temperatures within cities may provide better microclimatic conditions for the mostly thermo- and xerophilic wild bee species compared to the surrounding rural landscape (Fig. 4). Local land use is an important factor driving bee species richness and abundance (but see Dylewski et al., 2019), and higher bee species richness and abundance was observed on sites with a higher proportion of permeable substrate at the local scale along an urban-rural gradient (Choate et al., 2018). The importance of local factors is, however, likely to differ between bee species, depending on the commuting flight ranges and other life history traits. For example, Foster et al. (2017) did not find an effect of local land use on bumblebee species richness, and only marginal effects of land use on bumblebee abundance.

Fig. 4. Xerotherm urban habitat. Sun exposed nesting habitat in Berlin-Dahlem (Germany; left), with multiple nest entrances of a halictid bee species (right) in spring (Photos: André Krahner).
In addition to local factors, bee populations are influenced by landscape factors such as habitat configuration and connectivity, since bees generally use different partial habitats within the landscape. Therefore, mapping of land use within a study area on the landscape scale can help explain the distribution pattern of bee populations, in addition to local factors such as on-site floral richness and nesting opportunities. While classical mapping of landscape features on the ground is time consuming and resource intensive, landscape classification based on remote-sensing data is a feasible option to take landscape effects into consideration. These data are available for rural and urban landscapes at the same resolution, and can be further refined by additional data, e.g. higher-resolution data gathered for administrative purposes. Samuelson und Leadbeater (2018) propose a landscape classification protocol targeted at ecological research on pollinators, providing a case study along an urban-rural gradient.
The EU CORINE Land Cover inventory (European Union, Copernicus Land Monitoring Service, 2020a) offers a uniform classification of the most important types of ground cover. Some of these types are relevant for the distribution of bee populations, such as natural grasslands, forests, urban green spaces and surface water bodies. It is also possible to calculate the proportion of impervious substrate in a landscape from land use classification (Fortel et al., 2014). More directly, the proportion of impervious substrate can be used as a proxy for urbanization. Following this approach, Fitch et al. (2019a) linked changes in the observed sex ratio in bee communities to urbanization, and Schochet et al. (2016) found species specific effects of urbanization on the occurrence of different bumblebee species. Data on coverage of impervious substrate (Fig. 5) are readily available from the EU Copernicus program (European Union, Copernicus Land Monitoring Service, 2020b).
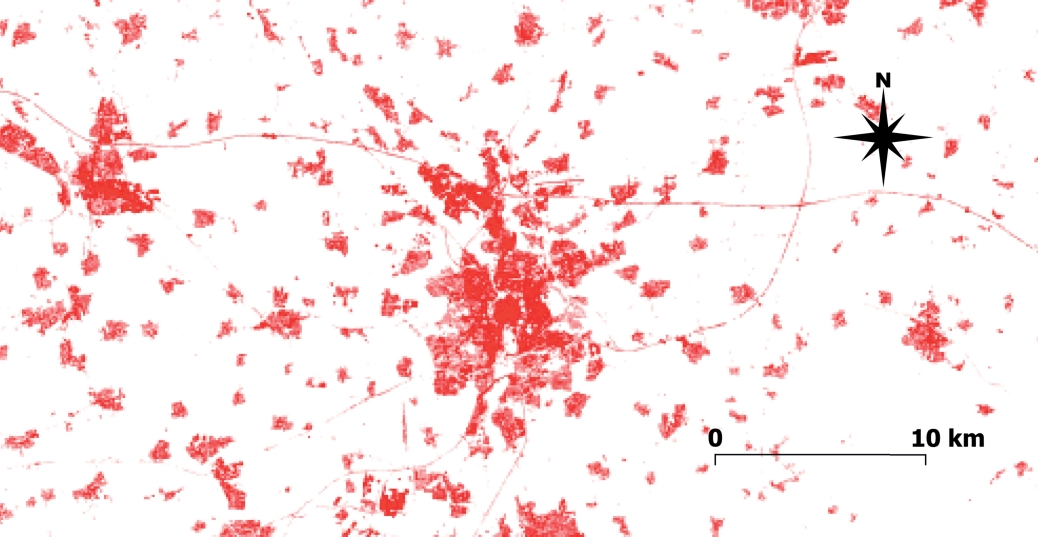
Fig. 5. Coverage of impervious substrate in Braunschweig and surroundings (Lower Saxony, Germany). Increasing coverage of impervious substrate is represented by increasing red colour. Data were obtained from the EU Copernicus program (European Union, Copernicus Land Monitoring Service, 2020b).
In heterogeneous urban landscapes, land use exerts direct and indirect effects on floral resources and the flower-visiting insect fauna dominated by bees (Matteson et al., 2013). Based on previous studies in urban settings, the most relevant landscape scale for bees is a radius of about 500–1000 m (but see Pardee und Philpott, 2014). Fortel et al. (2014) found a significant effect of impervious substrate on bee abundance and species richness at radii of 500 and 1000 m, but not at a radius of 2000 m. A 500 m radius has been chosen as the relevant landscape scale for the analysis of plant-pollinator networks (Geslin et al., 2013), effects of wildflower plantings on bee species richness and abundance in different agricultural landscapes (Batary et al., 2010; Grass et al., 2016), and the bee fauna visiting flowering lawn weeds along an urban-rural gradient (Larson et al., 2014). Significantly negative effects of percent agricultural cover in the surrounding landscape on bee species richness and phylogenetic diversity were observed at a 750 m radius (Grab et al., 2019). In an urban landscape, Lowenstein et al. (2014) identified the smallest tested radius of 100 m as the relevant scale with regard to the influence of land cover and sociometric variables, such as solar radiation, impervious substrate, tree canopy cover and population density, on bee abundance, species richness and community composition. However, in this study system there was a high degree of similarity in correlations between response and predictor variables over the analyzed radii from 100–1500 m (Lowenstein et al., 2014), rendering it impossible to identify the most relevant landscape scale from these results. Correlations between bee genus richness and landscape diversity peaked a radius of 1000 m (Theodorou et al., 2017).
The molecular tools to analyse genetic exchange within population such as microsatellites are under ongoing development (e.g. Mohra et al., 2000; Neumann und Seidelmann, 2006; Černá und Straka, 2012). Population structure has been investigated in several bee species (Darvill et al., 2006; Exeler et al., 2008; Černá et al., 2013), also in an urban context (López-Uribe et al., 2015). Using genome-wide SNPs, Theodorou et al. (2018) found little evidence of population structure in Bombus lapidarius associated with urbanization.
Differing sensitivity to microclimatic characteristics of urban habitats (Burdine und McCluney, 2019) and land use change (Cariveau und Winfree, 2015) might change the suitability for particular bee species to persist in the cities in the longer term. The evolutionary adaptations of bees to the urban environment have been studied with regard to wing morphology (Beasley et al., 2019), while MacIvor und Moore (2013) found indications for ecologically adaptive traits with regard to the use of plastics for nest construction in an urban environment.
A citizen scientist approach has been suggested as a plausible option for bee monitoring at morphospecies level, depending on volunteer training and engangement (Mason und Arathi, 2019). However, because bee species identification at species level is notoriously difficult and requires professional training, detailed information on the development of bee populations can only be gathered through a professional long-term monitoring program.
From the overview of recently published studies, several aspects are suggested that could guide research on bee promoting measures in urban settings towards study designs capable of resolving so far unresolved issues.
First of all, it is suggested that study designs are used which best represent a continuous degree of urbanization along a gradient which stretches from highly urbanized landscapes to landscapes dominated by intensive agriculture. Such studies will be very helpful in comparing results on bee promoting measures in agricultural landscapes (which are rather numerous) to urban settings (which are still encountered much less frequently). In this way, some lessons learned within the agricultural context can potentially be transferred to urban settings, and research on urban bee conservation can focus on the remaining open questions. Studies should stretch over multiple years, allow an estimation of activity density in the sampled habitats, and should incorporate before-after as well as with-without impact design, in order to compensate for annual fluctuations in population size and thus to estimate the impact of a measure on population development.
The relevant landscape scale should be identified for each study system and with regard to the research question, since the available studies indicate some (although relatively small) variation in the relevant scale. Modern GIS and publicly available data based on remote sensing allow an easy incorporation of landscape scale factors into ecological modelling. Methodological standardization needs to be tackled with regard to use of floral resources in trees by bees. Case studies on genetic structure of bee populations with regard to urbanization are still relatively scarce and represent only a tiny fraction of phylogenetic and functional bee diversity. Development of the relevant molecular methods has progressed over the past years, and the results from studies on genetic population structuring are promising with regard to future adaptations of molecular tools for documenting the success of bee promoting measures in terms of habitat connectivity.
The authors declare that they do not have any conflicts of interest.
Ahrné, K., J. Bengtsson, T. Elmqvist, 2009: Bumble Bees (Bombus spp) along a Gradient of Increasing Urbanization. Plos One 4 (5), e5574, DOI: 10.1371/journal.pone.0005574.
Baldock, K.C.R., 2020: Opportunities and threats for pollinator conservation in global towns and cities. Current Opinion in Insect Science, DOI: 10.1016/j.cois.2020.01.006.
Baldock, K.C.R., M.A. Goddard, D.M. Hicks, W.E. Kunin, N. Mitschunas, L.M. Osgathorpe, S.G. Potts, K.M. Robertson, A.V. Scott, G.N. Stone, I.P. Vaughan, J. Memmott, 2015: Where is the UK’s pollinator biodiversity? The importance of urban areas for flower-visiting insects. Proceedings of the Royal Society B-Biological Sciences 282 (1803), 20142849, DOI: 10.1098/rspb.2014.2849.
Balzan, M.V., G. Bocci, A.-C. Moonen, 2016: Utilisation of plant functional diversity in wildflower strips for the delivery of multiple agroecosystem services. Entomologia Experimentalis et Applicata 158 (3), 304–319, DOI: 10.1111/eea.12403.
Banaszak-Cibicka, W., M. Fliszkiewicz, A. Langowska, M. Żmihorski, 2018a: Body size and wing asymmetry in bees along an urbanization gradient. Apidologie 49 (3), 297–306, DOI: 10.1007/s13592-017-0554-y.
Banaszak-Cibicka, W., L. Twerd, M. Fliszkiewicz, K. Giejdasz, A. Langowska, 2018b: City parks vs. natural areas - is it possible to preserve a natural level of bee richness and abundance in a city park? Urban Ecosystems 21 (4), 599–613, DOI: 10.1007/s11252-018-0756-8.
Banaszak-Cibicka, W., M. Żmihorski, 2012: Wild bees along an urban gradient: winners and losers. Journal of Insect Conservation 16 (3), 331–343, DOI: 10.1007/s10841-011-9419-2.
Batary, P., A. Baldi, M. Sarospataki, F. Kohler, J. Verhulst, E. Knop, F. Herzog, D. Kleijn, 2010: Effect of conservation management on bees and insect-pollinated grassland plant communities in three European countries. Agriculture, Ecosystems and Environment 136, 35–39, DOI: 10.1016/j.agee.2009.11.004.
Bates, A.J., J.P. Sadler, A.J. Fairbrass, S.J. Falk, J.D. Hale, T.J. Matthews, 2011: Changing bee and hoverfly pollinator assemblages along an urban-rural gradient. PLoS ONE 6 (8), e23459, DOI: 10.1371/journal.pone.0023459.
Beasley, D.E., J.L. Fitzgerald, A. Fowler, K. Keleher, M.M. López-Uribe, R.R. Dunn, 2019: Do Bee Wings Adapt for Flight in Urban Environments? Southeastern Naturalist 18 (2), 183, DOI: 10.1656/058.018.0210.
Bellamy, C.C., A.P.N. van der Jagt, S. Barbour, M. Smith, D. Moseley, 2017: A spatial framework for targeting urban planning for pollinators and people with local stakeholders: A route to healthy, blossoming communities? Environmental Research 158, 255–268, DOI: 10.1016/j.envres.2017.06.023.
Bennett, A.B., S. Lovell, 2019: Landscape and local site variables differentially influence pollinators and pollination services in urban agricultural sites. PLoS ONE 14 (2), e0212034, DOI: 10.1371/journal.pone.0212034.
Blaauw, B.R., R. Isaacs, 2014: Flower plantings increase wild bee abundance and the pollination services provided to a pollination-dependent crop. Journal of Applied Ecology 51 (4), 890–898, DOI: 10.1111/1365-2664.12257.
Blackmore, L.M., D. Goulson, 2014: Evaluating the effectiveness of wildflower seed mixes for boosting floral diversity and bumblebee and hoverfly abundance in urban areas. Insect Conservation and Diversity 7 (5), 480–484, DOI: 10.1111/icad.12071.
Bundesministerium für Umwelt, Naturschutz, Bau und Reaktorsicherheit, 2017: Weißbuch Stadtgrün: Grün in der Stadt – Für eine lebenswerte Zukunft. Berlin.
Burdine, J.D., K.E. McCluney, 2019: Differential sensitivity of bees to urbanization-driven changes in body temperature and water content. Scientific Reports 9 (1), 1643, DOI: 10.1038/s41598-018-38338-0.
Burr, A., D.M. Hall, N. Schaeg, 2018: The perfect lawn: exploring neighborhood socio-cultural drivers for insect pollinator habitat. Urban Ecosystems 21 (6), 1123–1137, DOI: 10.1007/s11252-018-0798-y.
Byrne, F., J. delBarco-Trillo, 2019: The effect of management practices on bumblebee densities in hedgerow and grassland habitats. Basic and Applied Ecology 35, 28–33, DOI: 10.1016/j.baae.2018.11.004.
Cameron, S.A., S.A. Corbet, J.B. Whitfield, 2019: Bumble bees (Hymenoptera: Apidae: Bombus terrestris) collecting honeydew from the giant willow aphid (Hemiptera: Aphididae). Journal of Hymenoptera Research 68 (4), 75–83, DOI: 10.3897/jhr.68.30495.
Cane, J.H., R.L. Minckley, L.J. Kervin, T.H. Roulston, N.M. Williams, 2006: Complex Responses Within A Desert Bee Guild (Hymenoptera: Apiformes) To Urban Habitat Fragmentation. Ecological Applications 16 (2), 632–644, DOI: 10.1890/1051-0761(2006)016[0632:CRWADB]2.0.CO;2.
Cardoso, M.C., R.B. Gonçalves, 2018: Reduction by half: the impact on bees of 34 years of urbanization. Urban Ecosystems 21 (5), 943–949, DOI: 10.1007/s11252-018-0773-7.
Cariveau, D.P., R. Winfree, 2015: Causes of variation in wild bee responses to anthropogenic drivers. Current Opinion in Insect Science 10, 104–109, DOI: 10.1016/j.cois.2015.05.004.
Carvell, C., P. Westrich, W.R. Meek, R.F. Pywell, M. Nowakowski, 2006: Assessing the value of annual and perennial forage mixtures for bumblebees by direct observation and pollen analysis. Apidologie 37 (3), 326–340, DOI: 10.1051/apido:2006002.
Černá, K., J. Straka, 2012: Identification of 37 microsatellite loci for Anthophora plumipes (Hymenoptera: Apidae) using next generation sequencing and their utility in related species. European Journal of Entomology 109 (2), 155–160, DOI: 10.14411/eje.2012.020.
Černá, K., J. Straka, P. Munclinger, 2013: Population structure of pioneer specialist solitary bee Andrena vaga (Hymenoptera: Andrenidae) in central Europe: the effect of habitat fragmentation or evolutionary history? Conservation Genetics 14 (4), 875–883, DOI: 10.1007/s10592-013-0482-y.
Choate, B.A., P.L. Hickman, E.A. Moretti, 2018: Wild bee species abundance and richness across an urban–rural gradient. Journal of Insect Conservation 22 (3-4), 391–403, DOI: 10.1007/s10841-018-0068-6.
Collado, M.Á., D. Sol, I. Bartomeus, 2019: Bees use anthropogenic habitats despite strong natural habitat preferences. Diversity and Distributions 25 (6), 924–935, DOI: 10.1111/ddi.12899.
Darvill, B., J.S. Ellis, G.C. Lye, D. Goulson, 2006: Population structure and inbreeding in a rare and declining bumblebee, Bombus muscorum (Hymenoptera: Apidae). Molecular Ecology 15 (3), 601–611, DOI: 10.1111/j.1365-294X.2006.02797.x.
Dylewski, Ł., Ł. Maćkowiak, W. Banaszak-Cibicka, 2019: Are all urban green spaces a favourable habitat for pollinator communities? Bees, butterflies and hoverflies in different urban green areas. Ecological Entomology 22, 335, DOI: 10.1111/een.12744.
European Union, Copernicus Land Monitoring Service, 2020a: CORINE Land Cover. Access: 4th March 2020, URL: https://land.copernicus.eu/pan-european/corine-land-cover.
European Union, Copernicus Land Monitoring Service, 2020b: Imperviousness Density 2015 — Copernicus Land Monitoring Service. Access: 3rd March 2020, URL: https://land.copernicus.eu/pan-european/high-resolution-layers/imperviousness/status-maps/2015?tab=metadata.
Everaars, J., M.W. Strohbach, B. Gruber, C.F. Dormann, 2011: Microsite conditions dominate habitat selection of the red mason bee (Osmia bicornis, Hymenoptera: Megachilidae) in an urban environment: A case study from Leipzig, Germany. Landscape and Urban Planning 103 (1), 15–23, DOI: 10.1016/j.landurbplan.2011.05.008.
Exeler, N., A. Kratochwil, A. Hochkirch, 2008: Strong genetic exchange among populations of a specialist bee, Andrena vaga (Hymenoptera: Andrenidae). Conservation Genetics 9 (5), 1233–1241, DOI: 10.1007/s10592-007-9450-8.
Feltham, H., K. Park, J. Minderman, D. Goulson, 2015: Experimental evidence that wildflower strips increase pollinator visits to crops. Ecology and Evolution 5 (16), 3523–3530, DOI: 10.1002/ece3.1444.
Fischer, L.K., J. Eichfeld, I. Kowarik, S. Buchholz, 2016: Disentangling urban habitat and matrix effects on wild bee species. PeerJ 4, e2729, DOI: 10.7717/peerj.2729.
Fitch, G., P. Glaum, M.-C. Simao, C. Vaidya, J. Matthijs, B. Iuliano, I. Perfecto, 2019a: Changes in adult sex ratio in wild bee communities are linked to urbanization. Scientific Reports 9 (1), 3767, DOI: 10.1038/s41598-019-39601-8.
Fitch, G., C.J. Wilson, P. Glaum, C. Vaidya, M.-C. Simao, M.A. Jamieson, 2019b: Does urbanization favour exotic bee species? Implications for the conservation of native bees in cities. Biology Letters 15 (12), 20190574, DOI: 10.1098/rsbl.2019.0574.
Fortel, L., M. Henry, L. Guilbaud, A.L. Guirao, M. Kuhlmann, H. Mouret, O. Rollin, B.E. Vaissière, 2014: Decreasing abundance, increasing diversity and changing structure of the wild bee community (Hymenoptera: Anthophila) along an urbanization gradient. PLoS ONE 9 (8), e104679, DOI: 10.1371/journal.pone.0104679.
Fortel, L., M. Henry, L. Guilbaud, H. Mouret, B.E. Vaissière, 2016: Use of human-made nesting structures by wild bees in an urban environment. Journal of Insect Conservation 20 (2), 239–253, DOI: 10.1007/s10841-016-9857-y.
Foster, G., J. Bennett, T. Sparks, 2017: An assessment of bumblebee (Bombus spp) land use and floral preference in UK gardens and allotments cultivated for food. Urban Ecosystems 20 (2), 425–434, DOI: 10.1007/s11252-016-0604-7.
Fournier, B., D. Frey, M. Moretti, 2020: The origin of urban communities: From the regional species pool to community assemblages in city. Journal of Biogeography 21, 1, DOI: 10.1111/jbi.13772.
Geslin, B., B. Gauzens, E. Thébault, I. Dajoz, 2013: Plant pollinator networks along a gradient of urbanisation. PLoS ONE 8 (5), e63421, DOI: 10.1371/journal.pone.0063421.
Grab, H., M.G. Branstetter, N. Amon, K.R. Urban-Mead, M.G. Park, J. Gibbs, E.J. Blitzer, K. Poveda, G. Loeb, B.N. Danforth, 2019: Agriculturally dominated landscapes reduce bee phylogenetic diversity and pollination services. Science 363 (6424), 282–284, DOI: 10.1126/science.aat6016.
Grass, I., J. Albrecht, F. Jauker, T. Diekötter, D. Warzecha, V. Wolters, N. Farwig, 2016: Much more than bees—Wildflower plantings support highly diverse flower-visitor communities from complex to structurally simple agricultural landscapes. Agriculture Ecosystems & Environment 225, 45–53, DOI: 10.1016/j.agee.2016.04.001.
Gunnarsson, B., L. Federsel, 2014: Bumblebees in the city: abundance, species richness and diversity in two urban habitats. Journal of Insect Conservation 18 (6), 1185–1191, DOI: 10.1007/s10841-014-9729-2.
Haaland, C., R.E. Naisbit, L.-F. Bersier, 2011: Sown wildflower strips for insect conservation: a review. Insect Conservation & Diversity 4 (1), 60–80, DOI: 10.1111/j.1752-4598.2010.00098.x.
Hall, D.M., G.R. Camilo, R.K. Tonietto, J. Ollerton, K. Ahrne, M. Arduser, J.S. Ascher, K.C.R. Baldock, R. Fowler, G. Frankie, D. Goulson, B. Gunnarsson, M.E. Hanley, J.I. Jackson, G. Langellotto, D. Lowenstein, E.S. Minor, S.M. Philpott, S.G. Potts, M.H. Sirohi, E.M. Spevak, G.N. Stone, C.G. Threlfall, 2017: The city as a refuge for insect pollinators. Conservation Biology 31 (1), 24–29, DOI: 10.1111/cobi.12840.
Hamblin, A.L., E. Youngsteadt, S.D. Frank, 2018: Wild bee abundance declines with urban warming, regardless of floral density. Urban Ecosystems 21 (3), 419–428, DOI: 10.1007/s11252-018-0731-4.
Harrison, T., J. Gibbs, R. Winfree, 2019: Anthropogenic landscapes support fewer rare bee species. Landscape Ecology 34 (5), 967–978, DOI: 10.1007/s10980-017-0592-x.
Hausmann, S.L., J.S. Petermann, J. Rolff, 2016: Wild bees as pollinators of city trees. Insect Conservation and Diversity 9 (2), 97–107, DOI: 10.1111/icad.12145.
Heard, M.S., C. Carvell, N.L. Carreck, P. Rothery, J.L. Osborne, A.F.G. Bourke, 2007: Landscape context not patch size determines bumble-bee density on flower mixtures sown for agri-environment schemes. Biology Letters 3 (6), 638–641, DOI: 10.1098/rsbl.2007.0425.
Hennig, E.I., J. Ghazoul, 2012: Pollinating animals in the urban environment. Urban Ecosystems 15 (1), 149–166, DOI: 10.1007/s11252-011-0202-7.
Hernandez, J.L., G.W. Frankie, R.W. Thorp, 2009: Ecology of Urban Bees: A Review of Current Knowledge and Directions for Future Study. Cities and the Environment 2 (1), Article 3.
Hicks, D.M., P. Ouvrard, K.C.R. Baldock, M. Baude, M.A. Goddard, W.E. Kunin, N. Mitschunas, J. Memmott, H. Morse, M. Nikolitsi, L.M. Osgathorpe, S.G. Potts, K.M. Robertson, A.V. Scott, F. Sinclair, D.B. Westbury, G.N. Stone, 2016: Food for Pollinators: Quantifying the Nectar and Pollen Resources of Urban Flower Meadows. PLoS ONE 11 (6), DOI: 10.1371/journal.pone.0158117.
Hofmann, M.M., S.S. Renner, 2018: Bee species recorded between 1992 and 2017 from green roofs in Asia, Europe, and North America, with key characteristics and open research questions. Apidologie 49 (3), 307–313, DOI: 10.1007/s13592-017-0555-x.
Johansson, V., A. Koffman, M. Hedblom, G. Deboni, P. Andersson, 2018: Estimates of accessible food resources for pollinators in urban landscapes should take landscape friction into account. Ecosphere 9 (10), e02486, DOI: 10.1002/ecs2.2486.
Jönsson, A.M., J. Ekroos, J. Dänhardt, G.K.S. Andersson, O. Olsson, H.G. Smith, 2015: Sown flower strips in southern Sweden increase abundances of wild bees and hoverflies in the wider landscape. Biological Conservation 184, 51–58, DOI: 10.1016/j.biocon.2014.12.027.
Kratschmer, S., M. Kriechbaum, B. Pachinger, 2018: Buzzing on top: Linking wild bee diversity, abundance and traits with green roof qualities. Urban Ecosystems 21 (3), 429–446, DOI: 10.1007/s11252-017-0726-6.
Lane, I.G., J. Wolfin, E. Watkins, M. Spivak, 2019: Testing the Establishment of Eight Forbs in Mowed Lawns of Hard Fescue (Festuca brevipila) for Use in Pollinator Conservation. HortScience 54 (12), 2150–2155, DOI: 10.21273/HORTSCI14336-19.
Larson, J., A. Kesheimer, D. Potter, 2014: Pollinator assemblages on dandelions and white clover in urban and suburban lawns. Journal of Insect Conservation 18 (5), 863–873, DOI: 10.1007/s10841-014-9694-9.
Lerman, S.B., A.R. Contosta, J. Milam, C. Bang, 2018: To mow or to mow less: Lawn mowing frequency affects bee abundance and diversity in suburban yards. Biological Conservation 221, 160–174, DOI: 10.1016/j.biocon.2018.01.025.
López-Uribe, M.M., S.J. Morreale, C.K. Santiago, B.N. Danforth, 2015: Nest suitability, fine-scale population structure and male-mediated dispersal of a solitary ground nesting bee in an urban landscape. PLoS ONE 10 (5), e0125719, DOI: 10.1371/journal.pone.0125719.
Lowenstein, D.M., K.C. Matteson, E.S. Minor, 2015: Diversity of wild bees supports pollination services in an urbanized landscape. Oecologia 179 (3), 811–821, DOI: 10.1007/s00442-015-3389-0.
Lowenstein, D.M., K.C. Matteson, I. Xiao, A.M. Silva, E.S. Minor, 2014: Humans, bees, and pollination services in the city: the case of Chicago, IL (USA). Biodiversity and Conservation 23 (11), 2857-2874, DOI: 10.1007/s10531-014-0752-0.
Mach, B.M., D.A. Potter, 2018: Quantifying bee assemblages and attractiveness of flowering woody landscape plants for urban pollinator conservation. PLoS ONE 13 (12), e0208428, DOI: 10.1371/journal.pone.0208428.
MacIvor, J.S., 2016a: Building height matters: nesting activity of bees and wasps on vegetated roofs. Israel Journal of Ecology & Evolution 62 (1-2), 88–96, DOI: 10.1080/15659801.2015.1052635.
MacIvor, J.S., 2016b: DNA barcoding to identify leaf preference of leafcutting bees. Royal Society open science 3 (3), 150623, DOI: 10.1098/rsos.150623.
MacIvor, J.S., J.M. Cabral, L. Packer, 2014: Pollen specialization by solitary bees in an urban landscape. Urban Ecosystems 17 (1), 139–147, DOI: 10.1007/s11252-013-0321-4.
MacIvor, J.S., A.E. Moore, 2013: Bees collect polyurethane and polyethylene plastics as novel nest materials. Ecosphere 4 (12), art155, DOI: 10.1890/ES13-00308.1.
MacIvor, J.S., L. Packer, 2015: ‘Bee hotels’ as tools for native pollinator conservation: a premature verdict? PLoS ONE 10 (3), e0122126, DOI: 10.1371/journal.pone.0122126.
MacIvor, J.S., A. Ruttan, B. Salehi, 2015: Exotics on exotics: Pollen analysis of urban bees visiting Sedum on a green roof. Urban Ecosystems 18 (2), 419–430, DOI: 10.1007/s11252-014-0408-6.
Masierowska, M., E. Stawiarz, R. Rozwałka, 2018: Perennial ground cover plants as floral resources for urban pollinators: A case of Geranium species. Urban Forestry & Urban Greening 32, 185–194, DOI: 10.1016/j.ufug.2018.03.018.
Mason, L., H.S. Arathi, 2019: Assessing the efficacy of citizen scientists monitoring native bees in urban areas. Global Ecology and Conservation 17, e00561, DOI: 10.1016/j.gecco.2019.e00561.
Matteson, K.C., J.S. Ascher, G.A. Langellotto, 2008: Bee Richness and Abundance in New York City Urban Gardens. Annals of the Entomological Society of America 101 (1), 140–150, DOI: 10.1603/0013-8746(2008)101[140:BRAAIN]2.0.CO;2.
Matteson, K.C., J.B. Grace, E.S. Minor, 2013: Direct and indirect effects of land use on floral resources and flower-visiting insects across an urban landscape. Oikos 122 (5), 682–694, DOI: 10.1111/j.1600-0706.2012.20229.x.
Matteson, K.C., G.A. Langellotto, 2009: Bumble Bee Abundance in New York City Community Gardens: Implications for Urban Agriculture. Cities and the Environment 2 (1).
Matteson, K.C., G.A. Langellotto, 2011: Small scale additions of native plants fail to increase beneficial insect richness in urban gardens. Insect Conservation and Diversity 4 (2), 89–98, DOI: 10.1111/j.1752-4598.2010.00103.x.
McFrederick, Q.S., G. Lebuhn, 2006: Are urban parks refuges for bumble bees Bombus spp. (Hymenoptera: Apidae)? Biological Conservation 129 (3), 372–382, DOI: 10.1016/j.biocon.2005.11.004.
Mohra, C., M. Fellendorf, G. Segelbacher, R.J. Paxton, 2000: Dinucleotide microsatellite loci for Andrena vaga and other andrenid bees from non-enriched and CT-enriched libraries. Molecular Ecology 9 (12), 2189–2192, DOI: 10.1046/j.1365-294x.2000.01053-19.x.
Neumann, K., K. Seidelmann, 2006: Microsatellites for the inference of population structures in the Red Mason bee Osmia rufa (Hymenoptera, Megachilidae). Apidologie 37 (1), 75–83, DOI: 10.1051/apido:2005060.
Pardee, G.L., S.M. Philpott, 2014: Native plants are the bee’s knees: local and landscape predictors of bee richness and abundance in backyard gardens. Urban Ecosystems 17 (3), 641–659, DOI: 10.1007/s11252-014-0349-0.
Potter, C., N. de Vere, L.E. Jones, C.R. Ford, M.J. Hegarty, K.H. Hodder, A. Diaz, E.L. Franklin, 2019: Pollen metabarcoding reveals broad and species-specific resource use by urban bees. PeerJ 7, e5999, DOI: 10.7717/peerj.5999.
Razo-León, A.E., M. Vásquez-Bolaños, A. Muñoz-Urias, F.M. Huerta-Martínez, 2018: Changes in bee community structure (Hymenoptera, Apoidea) under three different land-use conditions. Journal of Hymenoptera Research 66 (1–2), 23–38, DOI: 10.3897/jhr.66.27367.
Rundlöf, M., O. Lundin, R. Bommarco, 2018: Annual flower strips support pollinators and potentially enhance red clover seed yield. Ecology and Evolution 8 (16), 7974–7985, DOI: 10.1002/ece3.4330.
Salisbury, A., J. Armitage, H. Bostock, J. Perry, M. Tatchell, K. Thompson, 2015: Enhancing gardens as habitats for flower-visiting aerial insects (pollinators): should we plant native or exotic species? Journal of Applied Ecology 52 (5), 1156–1164, DOI: 10.1111/1365-2664.12499.
Samuelson, A.E., R.J. Gill, M.J.F. Brown, E. Leadbeater, 2018: Lower bumblebee colony reproductive success in agricultural compared with urban environments. Proceedings of the Royal Society B-Biological Sciences 285 (1881), 20180807, DOI: 10.1098/rspb.2018.0807.
Samuelson, A.E., E. Leadbeater, 2018: A land classification protocol for pollinator ecology research: An urbanization case study. Ecology and Evolution 8 (11), 5598–5610, DOI: 10.1002/ece3.4087.
Scheper, J., R. Bommarco, A. Holzschuh, S.G. Potts, V. Riedinger, S.P.M. Roberts, M. Rundlöf, H.G. Smith, I. Steffan-Dewenter, J.B. Wickens, V.J. Wickens, D. Kleijn, 2015: Local and landscape-level floral resources explain effects of wildflower strips on wild bees across four European countries. Journal of Applied Ecology 52 (5), 1165–1175, DOI: 10.1111/1365-2664.12479.
Schochet, A.B., K.-L.J. Hung, D.A. Holway, 2016: Bumble bee species exhibit divergent responses to urbanisation in a Southern California landscape. Ecological Entomology 41 (6), 685–692, DOI: 10.1111/een.12343.
Senapathi, D., M.A. Goddard, W.E. Kunin, K.C.R. Baldock, 2017: Landscape impacts on pollinator communities in temperate systems: Evidence and knowledge gaps. Functional Ecology 31 (1), 26–37, DOI: 10.1111/1365-2435.12809.
Simao, M.-C.M., J. Matthijs, I. Perfecto, 2018: Experimental small-scale flower patches increase species density but not abundance of small urban bees. Journal of Applied Ecology 55 (4), 1759–1768, DOI: 10.1111/1365-2664.13085.
Sirohi, M.H., J. Jackson, M. Edwards, J. Ollerton, 2015: Diversity and abundance of solitary and primitively eusocial bees in an urban centre: A case study from Northampton (England). Journal of Insect Conservation 19 (3), 487–500, DOI: 10.1007/s10841-015-9769-2.
Sivakoff, F., S. Prajzner, M. Gardiner, 2018: Unique Bee Communities within Vacant Lots and Urban Farms Result from Variation in Surrounding Urbanization Intensity. Sustainability 10 (6), 1926, DOI: 10.3390/su10061926.
Somme, L., L. Moquet, M. Quinet, M. Vanderplanck, D. Michez, G. Lognay, A.-L. Jacquemart, 2016: Food in a row: Urban trees offer valuable floral resources to pollinating insects. Urban Ecosystems 19 (3), 1149–1161, DOI: 10.1007/s11252-016-0555-z.
Theodorou, P., K. Albig, R. Radzeviciute, J. Settele, O. Schweiger, T.E. Murray, R.J. Paxton, 2017: The structure of flower visitor networks in relation to pollination across an agricultural to urban gradient. Functional Ecology 31 (4), 838–847, DOI: 10.1111/1365-2435.12803.
Theodorou, P., R. Radzeviciute, J. Settele, O. Schweiger, T.E. Murray, R.J. Paxton, 2016: Pollination services enhanced with urbanization despite increasing pollinator parasitism. Proceedings of the Royal Society B-Biological Sciences 283 (1833), DOI: 10.1098/rspb.2016.0561.
Theodorou, P., R. Radzevičiūtė, B. Kahnt, A. Soro, I. Grosse, R.J. Paxton, 2018: Genome-wide single nucleotide polymorphism scan suggests adaptation to urbanization in an important pollinator, the red-tailed bumblebee (Bombus lapidarius L.). Proceedings of the Royal Society B-Biological Sciences 285 (1877), DOI: 10.1098/rspb.2017.2806.
Threlfall, C.G., K. Walker, N.S.G. Williams, A.K. Hahs, L. Mata, N. Stork, S.J. Livesley, 2015: The conservation value of urban green space habitats for Australian native bee communities. Biological Conservation 187, 240–248, DOI: 10.1016/j.biocon.2015.05.003.
Tommasi, D., A. Miro, H.A. Higo, M.L. Winston, 2004: Bee diversity and abundance in an urban setting. The Canadian Entomologist 136 (6), 851–869, DOI: 10.4039/n04-010.
Tonietto, R., J. Fant, J. Ascher, K. Ellis, D. Larkin, 2011: A comparison of bee communities of Chicago green roofs, parks and prairies. Landscape and Urban Planning 103 (1), 102–108, DOI: 10.1016/j.landurbplan.2011.07.004.
Tumminello, G., T.A. Volk, S.H. McArt, M.K. Fierke, 2018: Maximizing pollinator diversity in willow biomass plantings: A comparison between willow sexes and among pedigrees. Biomass and Bioenergy 117, 124–130, DOI: 10.1016/j.biombioe.2018.07.013.
Turo, K.J., M.M. Gardiner, 2019: From potential to practical: conserving bees in urban public green spaces. Frontiers in Ecology and the Environment 17 (3), 167–175, DOI: 10.1002/fee.2015.
Twerd, L., W. Banaszak-Cibicka, 2019: Wastelands: their attractiveness and importance for preserving the diversity of wild bees in urban areas. Journal of Insect Conservation 23 (3), 573–588, DOI: 10.1007/s10841-019-00148-8.
Vaidya, C., K. Fisher, J. Vandermeer, 2018: Colony Development and Reproductive Success of Bumblebees in an Urban Gradient. Sustainability 10 (6), 1936, DOI: 10.3390/su10061936.
van Heezik, Y.M., K.J.M. Dickinson, C. Freeman, 2012: Closing the Gap: Communicating to Change Gardening Practices in Support of Native Biodiversity in Urban Private Gardens. Ecology and Society 17 (1), DOI: 10.5751/es-04712-170134.
Warzecha, D., T. Diekötter, V. Wolters, F. Jauker, 2018: Attractiveness of wildflower mixtures for wild bees and hoverflies depends on some key plant species. Insect Conservation and Diversity 11 (1), 32–41, DOI: 10.1111/icad.12264.
Westrich, P., 2018: Die Wildbienen Deutschlands. Stuttgart, Ulmer.
Williams, N.M., K.L. Ward, N. Pope, R. Isaacs, J. Wilson, E.A. May, J. Ellis, J. Daniels, A. Pence, K. Ullmann, J. Peters, 2015: Native wildflower plantings support wild bee abundance and diversity in agricultural landscapes across the United States. Ecological Applications 25 (8), 2119–2131, DOI: 10.1890/14-1748.1.
Zajdel, B., M. Borański, K. Kucharska, A. Jojczyk, K. Brzezińska, 2019: Bumblebee Communities (Apidae, Bombini) in Urban Parks in Relation to Park Area and Other Characteristics. Polish Journal of Ecology 67 (1), 84, DOI: 10.3161/15052249PJE2019.67.1.007.