

First record of the leafhopper Hishimonus hamatus Kuoh, 1976 (Hemiptera: Cicadellidae) in Germany
Erstnachweis der Zikade Hishimonus hamatus Kuoh, 1976 (Hemiptera: Cicadellidae) in Deutschland
Journal für Kulturpflanzen, 72 (12). S. 586–589, 2020, ISSN 1867-0911, DOI: 10.5073/JfK.2020.12.04, Verlag Eugen Ulmer KG, Stuttgart
 | This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/deed.en). Dies ist ein Open-Access-Artikel, der unter den Bedingungen der Creative Commons Namensnennung 4.0 International Lizenz (CC BY 4.0) zur Verfügung gestellt wird (https://creativecommons.org/licenses/by/4.0/deed.de). |
When monitoring leafhoppers for phytoplasma vector management in the grapevine growing regions of Rhineland-Palatinate, Germany, some individuals of Hishimonus hamatus were collected at Neustadt a. d. Weinstraße. Specimen were caught by net as well as with Yellow Sticky Traps placed in shrubs. The species was determined visually and verified by molecular analysis. This is the first record of H. hamatus in Germany and also north of the Alp mountains denoting that this species is further expanding its territory in Europe.
Key words: Hishimonus, leafhopper monitoring, barcoding, cytochrome oxidase I (COI), first record in Germany
Während einer Erhebung in Weinbauregionen von Rheinland-Pfalz in Deutschland zum Vorkommen von Zikaden, die als Vektor für Phytoplasmen eine Rolle spielen, wurden bei Neustadt a. d. Weinstraße einige Individuen von Hishimonus hamatus gefangen. Die Tiere wurden mittels Handfang und Gelbtafel-Klebefallen an Sträuchern gesammelt. Die Bestimmung der Art erfolgte visuell und molekulargenetisch. Es ist der Erstnachweis von H. hamatus für Deutschland und nördlich der Alpen, was auf eine weitere Ausbreitung dieser Art in Europa hinweist.
Stichwörter: Hishimonus, Zikadenmonitoring, barcoding, cytochrome oxidase I (COI), Erstnachweis für Deutschland
The leafhopper Hishimonus hamatus is originally native to the Asian continent and this species obviously was introduced as an alien leafhopper to Europe. It was previously recorded in Slovenia (Seljak, 2013), Switzerland (Trivellone et al., 2015), Corse, France (Albre & Gibernau, 2019), and Italy (Quaglino et al., 2019). This indicates that the species has successfully established populations in Southern parts of Europe.
For the annual leafhopper monitoring approximately 40 Yellow Sticky Traps (Neudorff, Emmerthal, Germany) were distributed in grapevine growing regions of Rhineland-Palatinate and placed in or close to vineyards from July to October; the traps were checked fortnightly. In addition, leafhoppers were sampled from vegetation by net.
Genomic DNA was extracted with TNES-buffer in a non-destructive way to maintain the specimen (Sambrook & Russell, 2001; Peccoud et al., 2013). PCR was performed with the PuReTaq Ready-To-Go PCR Kit (GE Healthcare, Chicago, USA). Universal barcoding primers targeting the cytochrome oxidase I (COI) gene and PCR cycling conditions were applied as previously described (Folmer et al., 1994; Hebert et al., 2003). Obtained amplicons were sent for bi-directional sequencing (Eurofins Genomics, Ebersberg, Germany) and resulting sequences were compared by alignment and phylogenic analysis using Clustal Omega and MEGA-X (Sievers et al., 2011; Kumar et al., 2018) software, respectively. To test if phytoplasma were present in H. hamatus, PCR with the general primers P1 and P7 was performed (Lorenz et al., 1995).
In order to assess phytoplasma vectors in the grapevine growing areas during the leafhopper monitoring in Rhineland-Palatinate, Germany, six individuals of H. hamatus were collected at a house garden close to vineyards located at the outskirts of Neustadt a. d. Weinstraße from July to September 2020. Four adult individuals were caught with Yellow Sticky Traps placed in a forsythia (Forsythia × intermedia) shrub or by net on golden privet (Ligustrum ovalifolium 'Aureum') leaves. Furthermore, two nymphs were collected with a net from golden privet leaves. The nymphs were kept on Ligustrum leaves in captivity for further development and final molting to the adult stage (Fig. 1). Collected individuals were determined visually as H. hamatus, which was confirmed on the molecular level (identifier at BOLD system: GERNW001PW, RLP001–20 and GERNW002PW, RLP002–20) (Fig. 2). Compared to the reference nucleotide (nt) sequence CORSE069–19 (658 nt) from an individual from France available at BOLD system the obtained COI core sequences share 99% identity with 3 and 4 missmatches for RLP001–20 (670 nt) and RLP002–20 (644 nt), respectively.
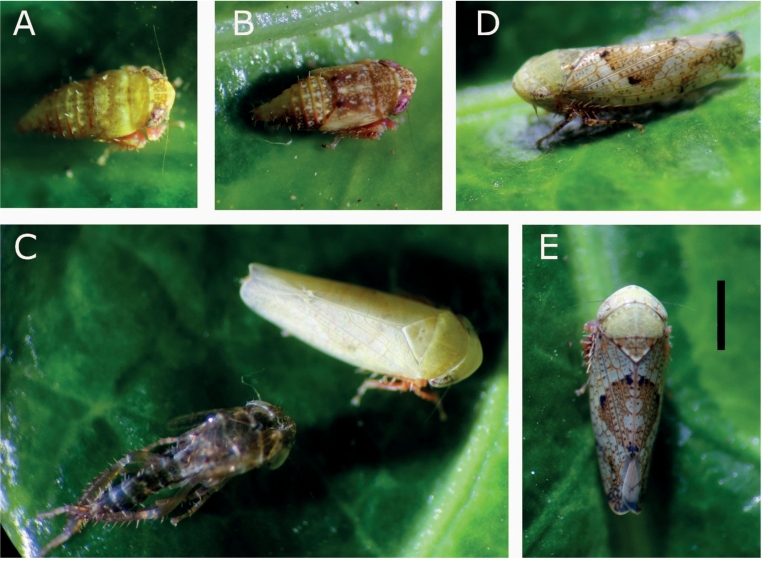
Fig. 1. Hishimonus hamatus collected on Ligustrum in Germany. A) 4th and B) 5th instar nymphs, C) freshly molted and still pale imago next to exuvia, D) and E) fully colored imago. Scalebar = 1 mm.
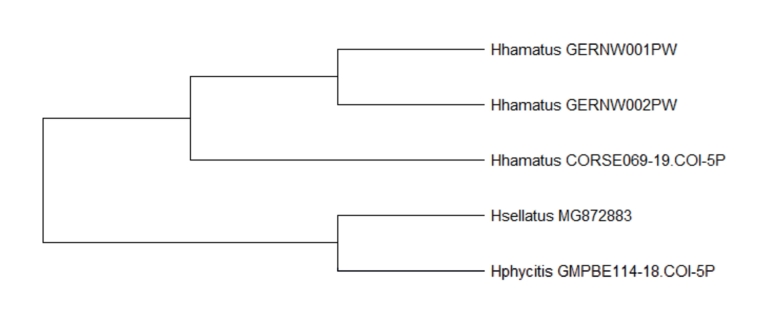
Fig. 2. Phylogenic tree displays maximum likelihood alignment of translated COI core sequences from German individuals and sequences from different Hishimonus species available in databases. The species names and the corresponding identifiers are indicated.
The presented data reveal the first verified record of H. hamatus in Germany and north of the Alps and the finding of developing nymphs confirms that the species is successfully propagating. A further random search of the immediate surroundings did not yield in more individuals. However, additional findings that still need to be verified on species level are expected from other areas in southwest Germany (personal communication, H. Nickel). How and when H. hamatus reached Germany is unknown at present.
It is noted that H. hamatus is polyphagous and feeds on various woody plants (Seljak, 2013). Due to the limited insect material available, only a preliminary study could be performed. At a feeding test a 5th instar nymph immediately accepted a grapevine leaf without further probing and fed on that for several hours before switching to the in parallel offered Ligustrum leaves from which it was collected before. This suggests that H. hamatus may accept an even wider range of plant species as food source then previously described. Regarding phytosanitary issues, it would be important to know if H. hamatus can act as a vector for phytoplasmas. A potential risk exists that phytoplasmas present in European plants and which may be compatible to H. hamatus might be taken up and spread by this leafhopper. However, at present there is no proof that H. hamatus transmits phytoplasma and an initial PCR test for detecting different phytoplasmas in H. hamatus DNA extracts (n = 2) turned out to be negative (data not shown). To obtain reliable data, representative studies with an appropriate number of individuals and transmission experiments are necessary.
It should be considered that introduced alien leafhopper species are candidates to become a vector, as they may be able to pick up compatible native phytoplasma from host plants and spread those (Malembic-Maher et al., 2020). A famous example for an alien species acting as vector is the leafhopper Scaphoideus titanus (Deltocephalinae) from America that was introduced in Europe and spreads the Flavescence dorée phytoplasma in vineyards (Chuche & Thiéry, 2014). Further, it was experimentally demonstrated that Orientus ishidae (Deltocephalinae), a polyphagous species originally native to Asia accidentally introduced to Europe, can be infected with 16SrV-group phytoplasmas and it is able to transmit those to grapevine (Mehle et al., 2010; Lessio et al., 2016). A similar scenario as found for O. ishidae cannot be excluded for H. hamatus yet because the genus Hishimonus also belongs to the subfamily Deltocephalinae and the species H. phycitis and H. sellatus are reported to transmit phytoplasmas (Tanaka et al., 2000; Cha & Han, 2002; EFSA, 2017). Conclusively, the occurrence of H. hamatus and the expansion of its territory in Europe should be further observed to identify any risk for horticultural or native plants as early as possible and to take appropriate measures if necessary.
Thanks to Herbert Nickel for information and discussion about a possible further occurrence of H. hamatus in Germany.
The author declares no conflict of interest.
Albre, J., M. Gibernau, 2019: Diversity and temporal variations of the Hemiptera Auchenorrhyncha fauna in the Ajaccio region (France, Corsica). Annales de la Société entomologique de France (N.S.) 55 (6), 497-508, DOI: 10.1080/00379271.2019.1688189.
Cha, B., S. Han, 2002: Genetic similarity between Jujube Witches´ broom and Mulberry dwarf phytoplasmas transmitted by Hishimonus sellatus Uhler. The Plant Pathology Journal 18, DOI: 10.5423/PPJ.2002.18.2.098.
Chuche, J., D. Thiéry, 2014: Biology and ecology of the Flavescence dorée vector Scaphoideus titanus: a review. Agronomy for Sustainable Development 34 (2), 381-340.
EFSA, 2017: pest cetagorisation of Witches´ broom disease of lime (Citrus aurantifolia) phytoplasma. EFSA Journal 15 (10), 22 pages.
Folmer, O., M. Black, W. Hoeh, R. Lutz, R. Vrijenhoek, 1994: DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3 (5), 294-299.
Hebert, P.D.N., A. Cywinska, S.L. Ball, J.R. deWaard, 2003: Biological identifications through DNA barcodes. Proceedings of the Royal Society of London B 270, 313-321.
Kumar, S., G. Stecher, M. Li, C. Knyaz, K. Tamura, 2018: MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35 (6), 1547-1549, DOI: 10.1093/molbev/msy096.
Lessio, F., L. Picciau, E. Gonella, M. Mandrioli, F. Tota, A. Alma, 2016: The mosaic leafhopper Orientus ishidae: host plants, spatial distribution, infectivity, and transmission of 16SrV phytoplasmas to vines. Bulletin of Insectology 69 (2), 277-289.
Lorenz, K.H., B. Schneider, U. Ahrens, E. Seemüller, 1995: Detection of the apple proliferation and pear decline Phytoplasmas by PCR amplification of ribosomal and nonribosomal DNA. Phytopathology 85 (7), 771-776.
Malembic-Maher, S., D. Desque, D. Khalil, P. Salar, B. Bergey, J.L. Danet, S. Duret, M.P. Dubrana-Ourabah, L. Beven, I. Ember, Z. Acs, M. Della Bartola, A. Materazzi, L. Filippin, S. Krnjajic, O. Krstic, I. Tosevski, F. Lang, B. Jarausch, M. Kolber, J. Jovic, E. Angelini, N. Arricau-Bouvery, M. Maixner, X. Foissac, 2020: When a Palearctic bacterium meets a Nearctic insect vector: Genetic and ecological insights into the emergence of the grapevine Flavescence doree epidemics in Europe. PLOS Pathogens 16 (3), e1007967, DOI: 10.1371/journal.ppat.1007967.
Mehle, N., G. Seljak, M. Rupar, M. Ravnikar, M. Dermastia, 2010: The first detection of a phytoplasma from the 16SrV (Elm yellows) group in the mosaic leafhopper Orientus ishidae. New Disease Reports 22, 11, DOI: 10.5197/j.2044-0588.2010.022.011.
Peccoud, J., G. Labonne, N. Sauvion, 2013: Molecular tests to assign individuals within the Cacospylla pruni complex. PLoS One 8 (8), e72454.
Quaglino, F., F. Sanna, A. Moussa, M. Faccincani, A. Passera, P. Casati, P.A. Bianco, N. Mori, 2019: Identification and ecology of alternative insect vectors of 'Candidatus Phytoplasma solani' to grapevine. Scientific Reports 9 (1), 19522, DOI: 10.1038/s41598-019-56076-9.
Sambrook, J., D.W. Russell, 2001: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, New York, Cold Spring Harbor Laboratory Press.
Seljak, G., 2013: Hishimonus hamatus Kuoh (Hemiptera: Cicadellidae): A new alien leafhopper in Europe. Acta Entomologica Slovenica 21 (2), 123-130.
Sievers, F., A. Wilm, D. Dineen, T.J. Gibson, K. Karplus, W. Li, R. Lopez, H. McWilliam, M. Remmert, J. Soding, J.D. Thompson, D.G. Higgins, 2011: Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molecular Systems Biology 7, 539, DOI: 10.1038/msb.2011.75.
Tanaka, M., S. Osada, I. Matsuda, 2000: Transmission of Rhus (Rhus javanica L.) yellows by Hishimonus sellatus and host range of the causal phytoplasma. Journal of General Plant Pathology 66, 323-326, DOI: 10.1007/PL00012972.
Trivellone, V., E. Knop, T. Turrini, A. Andrey, J.-Y. Humbert, G. Kunz, 2015: New and remarkable leafhoppers and planthoppers (Hemiptera: Auchenorrhyncha) from Switzerland. MITTEILUNGEN DER SCHWEIZERISCHEN ENTOMOLOGISCHEN GESELLSCHAFT/BULLETIN DE LA SOCIETE ENTOMOLOGIQUE SUISSE 88, 273-284.