

Soil transmission studies with four pome fruit viruses
Untersuchungen zur Bodenübertragung von vier Kernobstviren
Journal für Kulturpflanzen, 73 (3-4). S. 72–82, 2021, ISSN 1867-0911, DOI: 10.5073/JfK.2021.03-04.02, Verlag Eugen Ulmer KG, Stuttgart
 | This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/deed.en). Dies ist ein Open-Access-Artikel, der unter den Bedingungen der Creative Commons Namensnennung 4.0 International Lizenz (CC BY 4.0) zur Verfügung gestellt wird (https://creativecommons.org/licenses/by/4.0/deed.de). |
Soil transmission of the pome fruit viruses Apple chlorotic leafspot virus (ACLSV), Apple mosaic virus (ApMV), Apple stem grooving virus (ASGV) and Apple stem pitting virus (ASPV) was studied in pot and field trials. Non-inoculated recipient trees of apple seedlings were placed between inoculated donor trees, which were double-infected with either ACLSV/ApMV or ASGV/ASPV, and tested repeatedly by reverse transcription PCR (RT-PCR) for infections. In the first pot trial, a high potential for virus spread was demonstrated. 46% of the recipient trees were infected either by one or two of the viruses after four years. In a second pot trial, where the root contact between donor and recipient trees was restricted by a fine-meshed net, only a single virus transmission was detected. In a third pot trial, non-inoculated trees were watered with a soil eluate generated in the first pot trial, but no virus transmission was recorded. Moreover, no virions could be detected in the eluates generated from the first and second pot trial. The field trial consisted of two parallel rows of apple seedlings, where only one row was interspersed with virus-inoculated trees. In this row a total of five non-inoculated plants out of 44 (11%) tested positive for one or both viruses after 46 months. In the other row, which was planted one year later, only a single infection was found. The earliest virus transmissions in the field- and in the first pot trial were recorded after 17.5 months of exposure, however, most infections occurred between the third and fourth year. ACLSV was transmitted about twice as often as ApMV, whereas the rate for ASGV and ASPV was the same. The number of single and double virus transmissions was equal in the field, but in the first pot experiment single infections were recorded almost twice as often as double infections. There was no evidence that plant-parasitic nematodes were involved in virus transmission. The results of this study suggest that the transmission of all four viruses can be attributed to root grafting.
Key words: Apple chlorotic leafspot virus, Apple mosaic virus, Apple stem grooving virus, Apple stem pitting virus, apple seedling, transmission rate, root grafting, soil water, RT-PCR
In Topf- und Feldversuchen wurde die Bodenübertragung der Kernobstviren Chlorotisches Blattfleckenvirus des Apfels (ACLSV), Apfelmosaikvirus (ApMV), Stammfurchungsvirus des Apfels (ASGV) und Stammnarbungsvirus des Apfels (ASPV) untersucht. Nicht-inokulierte Bäume von Apfelsämlingen wurden zwischen inokulierte Bäume, die entweder mit ACLSV/ApMV oder ASGV/ASPV doppelt infiziert waren, gepflanzt und mit der Reversen Transkriptase-PCR (RT-PCR) wiederholt auf Infektionen getestet. Im ersten Topfversuch konnte ein hohes Ausbreitungspotenzial der Viren festgestellt werden. 46 % der nicht-inokulierten Bäume waren nach vier Jahren mit einem oder beiden der Viren infiziert. In einem zweiten Topfversuch, in welchem der Wurzelkontakt zwischen inokulierten und nicht-inokulierten Bäumen durch ein feinmaschiges Netz eingeschränkt war, wurde dagegen nur eine einzige Virusübertragung nachgewiesen. In einem dritten Topfversuch, in welchem nicht-inokulierte Bäume mit einem Bodeneluat bewässert wurden, welches aus dem ersten Topfversuch stammte, konnte keine Virusübertragung festgestellt werden. Darüber hinaus ließen sich in Eluaten aus dem ersten und zweiten Topfversuch keine Virionen nachweisen. Im Feldversuch mit zwei parallelen Reihen von Sämlingen, von denen nur eine Reihe mit virus-inokulierten Bäumen durchsetzt war, waren nach 46 Monaten fünf von 44 (11 %) der nicht-inokulierten Bäume in der durchsetzten Reihe mit einem oder zwei Viren infiziert. In der anderen Reihe, die ein Jahr später gepflanzt wurde, wurde nur eine einzige Infektion gefunden. Sowohl im Feld- als auch im ersten Topfversuch wurden erste Virusübertragungen nach 17,5 Monaten Expositionszeit festgestellt, die meisten fanden jedoch zwischen dem dritten und vierten Jahr statt. ACLSV wurde etwa doppelt so oft wie ApMV übertragen, die Übertragungsraten für ASGV und ASPV waren dagegen gleich. Einzel- und Doppelübertragungen traten im Feldversuch gleich häufig auf, im ersten Topfversuch waren Einzelinfektionen jedoch nahezu zweimal so häufig wie Doppelinfektionen. Für eine Beteiligung pflanzenparasitärer Nematoden an den Virusübertragungen fanden sich keine Hinweise. Die Ergebnisse dieser Studie legen den Schluss nahe, dass die Übertragung aller vier Viren auf Wurzelverwachsungen zurückgeführt werden kann.
Stichwörter: Chlorotisches Blattfleckenvirus des Apfels, Apfelmosaikvirus, Stammfurchungsvirus des Apfels, Stammnarbungsvirus des Apfels, Apfelsämling, Übertragungsrate, Bodenwasser, Wurzelverwachsung, RT-PCR
Apple chlorotic leaf spot virus (ACLSV), Apple mosaic virus (ApMV), Apple stem grooving virus (ASGV) and Apple stem pitting virus (ASPV) are the most common viruses on pome fruit. Due to their economic importance they are subjected to national and international certification guidelines such as that of the European Union for healthy fruit planting material (Anonymous, 2014). Efficient control of viruses is only possible when all transmission pathways are known. For apple, the four above mentioned viruses are commonly considered not to be seed, pollen or vector transmissible and only some are, with difficulties, mechanically transmissible to certain herbaceous plant species (Jelkmann & Paunovic 2011; Massart et al., 2011; Petrzik & Lenz, 2011; Yaegashi et al., 2011; Grimová et al., 2016). Spread of these viruses is therefore likely to occur mainly by vegetative propagation of infected plant material. However, in the field, natural spreads of infection have been observed in apple orchards and among other host plant species for some of these viruses. Root graftings were considered to be involved (Petrzik & Lenz, 2011; Yaegashi et al., 2011; Grimová et al., 2016). Natural root grafts are known for many horticultural crops (Graham & Bormann, 1966) and transmission of viruses and other pathogens by this phenomenon was shown for a number of other plant species (Epstein, 1978). ApMV for example, has been shown to be transmitted by root grafts between roses (Golino et al., 2007) and also phloem-inhabiting bacteria like ‘Candidatus Phytoplasma mali’, have been transmitted between apple trees this way (Ciccotti et al., 2007; Lešnik et al., 2008). Beside root grafts, other transmission pathways were occasionally demonstrated or suspected to be involved in the natural spread of some of these pome fruit viruses. A direct transmission by leaf or branch contact or by insect vectors was considered for ACLSV on apple (McCrum, 1965). Foliar contacts (Akbaş & Değirmenci, 2009) as well as an unknown slow-moving arthropod vector (Arli Sökmen et al., 2005) were suggested as a possible pathway for ApMV in hazelnut orchards. Mechanical transmission by foliar contact as well as simulated shoot pruning with scalpel blades was demonstrated for this virus on hop plants (Pethybridge et al., 2002). According to Roberts (2014) vectorless soil-borne viruses are supposed to infect plants through microscopic root wounds. Transmission of ACLSV between Chenopodium quinoa plants by the nematode Eudorylaimus sp. was reported by Fritzsche & Kegler (1968). The possible implication of water for virus transmission, however, received little attention, although a number of plant-pathogenic viruses have been detected in rivers, streams, lakes, drainage waters, wastewater and irrigation systems (reviewed by Koenig, 1986; Sevik, 2011; Mehle & Ravnikar, 2012; Bačnik et al., 2020). Virus release from infected roots was demonstrated for a number of viruses including Southern bean mosaic virus, Potato virus Y, Tobacco mosaic virus and some members of the Tombusviridae family (Smith et al., 1969; Kegler et al., 1980, Koenig, 1986; Teakle, 1986; Mehle et al., 2014; Roberts, 2014). Overall, however, little is known about the relevance of this pathway in the abiotic soil transmission of pome fruit viruses. Moreover, detailed studies about the potential and dynamic of virus dissemination in the soil have been sparsely reported (McCrum, 1965; Dhingra, 1972). A better understanding of the time course and extent of this process will be essential to estimate more precisely the threat that emanate from virus-infected trees to healthy trees in their vicinity. This would be of particular importance for a propagation orchard, where infected trees are detected after planting and retesting of the stock is needed for virus eradication.
To investigate the dynamic of pome fruit virus transmission in the soil environment, experiments with artificially virus-inoculated and healthy trees of the common apple rootstock ‘Bittenfelder’ were conducted in pot and field experiments. Focus was placed on possible virus transmission by root contacts and soil water.
All trials were conducted with certified 1-year-old apple seedlings of the cultivar ‘Bittenfelder’, which is widely used as a rootstock for scion wood production in commercial mother orchards. Virus-infected donor trees were generated in March 2013 by inoculation of seedlings with bark chips originating from virus-infected apple trees of the virus collection of the Federal Research Centre for Cultivated Plants, Institute for Plant Protection in Fruit Crops and Viticulture in Dossenheim. The isolates ASGV Gambach (41/85), ASPV PB66 (40/85), ACLSV Uhl (38/85) and ApMV (41/87) were used for double infections in the combination ASGV/ASPV or ACLSV/ApMV. The origin of the isolates is described by Menzel et al. (2002) and Zhang & Jelkmann (2017). All inoculated ‘Bittenfelder’ individuals used in the transmission trials are referred hereafter as “donor” trees and all non-inoculated ‘Bittenfelder’ individuals as “recipient” trees. The donor trees were tested three months post inoculation by RT-PCR for the presence of viruses and in case of negative results for one or both viruses the test was repeated after seven and nine months. The ASGV inoculated trees, which tested negative after the first test, were re-inoculated by two bark chips in August 2013. Donor trees from the trials which still tested negative after December 2013 were replaced by confirmed virus-positive trees. By April 2014 the double infection of all donor trees was confirmed. All trees were strongly pruned in the spring of 2014 and in the following years, in a manner similar to the pruning of mother trees for scion production. Scissor blades were disinfected every time before pruning another tree by immersing the blades in 70% ethanol for at least 30 seconds.
Although rootstocks grown from apple seeds are generally considered virus-free (see introduction), samples from seven randomly selected non-inoculated trees were screened for the absence of ACLSV, ApMV, ASGV and ASPV viruses by reverse transcriptase-PCR (RT-PCR). The recipient trees of the different experiments were tested for the presence of the viruses at various times post planting (exposure time) according to Table 1.
Table 1. Sampling intervals for recipient trees in the various experiments
|
| Months of exposure | ||||||||||||||||||||
|
| 3 | 6 | 6.5 | 10 | 13.5 | 15 | 17.5 | 19 | 21 | 22 | 25 | 26.5 | 27 | 30.5 | 31 | 32.5 | 36 | 36.5 | 44 | 46 | 48 |
Experiment | Start |
| ||||||||||||||||||||
Pot I | 03/13 |
|
| x |
|
|
| x |
|
|
|
| x |
|
| x |
|
| x |
|
| x |
Pot II | 07/13 | x |
|
|
| x |
|
|
|
| x |
|
| x |
|
| x |
|
| x |
|
|
Pot III | 07/14 |
|
|
| x |
| x |
|
| x |
|
|
|
|
|
|
|
|
|
|
|
|
Field row A | 03/13 |
|
| x |
|
|
| x |
|
|
|
| x |
| x |
|
| x |
|
| x |
|
Field row B | 03/14 |
| x |
|
|
|
|
| x |
|
| x |
|
|
|
|
| x |
|
|
|
|
Five leaves or shoot pieces were randomly collected from each tree, stored at + 8°C and processed within one day to three weeks. Total nucleic acids were extracted from the pooled samples, which consisted either of leaf stalks with some leaf tissue or bark chips containing phloem and buds, using the silica capture method described by Menzel et al. (2002), except 300 mg of tissue was used for extraction at a ratio of 1:10 (w/v) with grinding buffer instead of 100 mg. RT-PCR was conducted according to the method described by Menzel et al. (2002), except that the final volume of the reaction mixture was 25 μl instead of 50 μl. Accordingly 0.5 μl instead of 1.0 μl total nucleic acids extract was used. PCR reactions were run as duplex assay for ACLSV and ApMV and individually for ASGV and ASPV.
For all pot trials a sieved (5 mm mesh size) soil from the experimental field station at the Center for Agricultural Technology Augustenberg (LTZ) in Rheinstetten-Forchheim was used. Soil parameters were analysed by the LTZ division for inorganic investigations according to VDLUFA method manual (Anonymous, 1991) and characterized as a strong loamy sand, with fractions of 2.2% humus and 12.2% clay, pH 6.3. The soil was heat-treated for at least 20 min. at 70°C and stored dry until the beginning of the experiments.
A grit layer about 2.5 cm off the ground prevented waterlogging in the pots. All pots were placed outdoor on a weed-impermeable plastic foil and were roofed by a foil tunnel with open side walls in the first vegetation period. To improve growth, the pots were then kept under a plastic net roof protecting against excessive sunlight and hail. The plants were supplied by drip irrigation, admixed with liquid fertilizers Hakaphos and Novatec in a concentration recommended by the manufacturer (COMPO Company, Münster, Germany). During wintertime, the pots were kept in an unheated greenhouse. Weeds growing in the containers were removed by hand, however, the pots were not kept completely weed-free. Three experimental set-ups were conducted using donor trees and recipient trees to test virus transmission.
In experiment I a donor tree, about two weeks post inoculation, was placed in the centre of a 20-l plastic container containing two recipient trees (Fig. 1A). Six replicates each were prepared for the combinations ASGV/ASPV and ACLSV/ApMV. This experiment was also used to produce a soil eluate, which was needed for experiment III. The eluate was generated in July 2014. Each container was watered with 1.25 – 1.5 l of deionized water and the flow-through (about 0.8 – 0.9 l) collected. From each eluate an aliquot of about 0.4 l was stored at –18°C for later analysis of virus presence. The remaining eluates from the pots of both virus combinations (12 in total) were pooled, resulting in a total volume of about 5.5 l. Approximately 0.75 l aliquots were used to irrigate each of the containers from experiment III.

Fig. 1. Position of the donor tree (centre) and recipient trees (adjacent) in pot experiment I (A) and experiment II (B).
In experiment II, the effects of restricting root contact between the donor tree and the recipient trees were studied. This trial commenced in July 2013. The donor tree was grown in a 10-l plastic container with 100 evenly distributed 2-mm holes. The outside of the container was completely covered with a polyamide net of 250 μm mesh width (specification: Type PA 250/39, 6.6 monofil, 280 μm thickness, 1100 N/5 cm tear resistance, 33/40% tear distension, SAATI Company, Raesfeld, Germany). On the inside, the container was coated by two layers of different polyamide nets. The one close to the pot wall had a mesh width of 80 μm (specification: Type PA 80/32, 6.6 monofil, 110 μm thickness, 500/535 N/5 cm tear resistance, 43% tear distension, SAATI Company, Raesfeld, Germany) overlaid by a net as used for the outside. The stronger tear resistance of the outer- and innermost layer was intended to protect the finer mesh net against structural damage by the expansion of thicker roots from both directions. The potted donor tree was placed in the centre of a second rectangular plastic container of about 45-l volume with one recipient tree on each side (Fig. 1B). The number of replicates was similar to that of experiment I. To examine the soil for the presence of viruses, each pot was washed out with 1.25 l of tap water two months after planting. The eluates (about 0.8 l per pot) were collected and stored at –18°C until analysis, as well as a sample of tap water, which served as control. In October 2016, the 80 μm mesh size polyamide net was examined for root passages in two of the 12 pots. Therefore, the trees including the nets were carefully lifted off the pot and the nets were gently rinsed with water to remove soil particles and loose root fragments. After the nets were air dried, a microscopic examination for root passages was performed. After which the plants including the nets were placed back into the pots.
In experiment III, the infection potential of soil eluates generated from experiment I was tested. Two recipient trees were planted in a 20-l container in March 2013 in six replicates. In July 2014, each container was irrigated with 0.75 l of eluate obtained by the soil washout from experiment I. Leaf samples of the trees were monitored for viruses according to Table 1.
Analysis of soil eluates from experiment I and II for the presence of viruses was done with the pot replicate numbers 2, 3 and 4 for the respective viruses (12 samples in total) and was performed as follows: After thawing at room temperature, the bottles containing the soil eluates were shaken by hand for about 10 seconds to disperse the soil sediment. An aliquot of 200 ml from each bottle was centrifuged for 20 min at 6641 × g at room temperature to sediment the soil particles. The supernatant was decanted and centrifuged at 159,000 × g at 12°C for virus sedimentation. The centrifugation time was 7.5 h but for procedural purposes overnight runs were also performed. The decanted supernatant was replaced by fresh soil eluate until the complete eluate was processed. The pellet of each tube was suspended in 3 ml of silica grinding buffer. The RNA was extracted from the samples as described above.
This experiment was carried out on a plot at the fruit experimental station of the LTZ, which soil surface was inclined by about 5%. Thirty-two 1-year-old apple seedlings cv. ‘Bittenfelder’ were planted with a 0.6 m spacing in a continuous row across the slope in March 2013. Every third tree (10 in total) was inoculated before by bark chips with ASGV and ASPV (Fig. 4, row A). An analogous experiment with ACLSV and ApMV inoculated donor trees extended the row. Both parts were separated by a space of about 2 m. In a parallel row 1.35 m downhill, 64 1-year-old certified apple seedlings cv. ‘Bittenfelder’ were planted one year later (Fig. 4, row B). The distance between these trees was the same as above. The recipient trees were tested for the presence of viruses according to Table 1.
The dimension of the root system was examined for two specimens of ‘Bittenfelder’ seedlings 12 and 42 months post planting. For the first examination, inoculated but ASGV-negative trees were unearthed. For the second examination, the top layer of soil between two trees was removed until the primary roots were clearly visible and their lengths estimated. The complete dimensions including the fine root systems were not determined. Some characteristic soil parameters were as follows: Silt loam, 2.8% humus, 13.1% clay, pH 6.9.
Soil samples from pots of experiment I and II and from one field site, where virus-transmissions were recorded until August 2016, were analysed for the presence of plant parasitic and virus-transmitting nematodes. Soil samples were taken using a drill stick. From each pot three core samples, spanning the surface to the pot bottom, were collected and mixed, resulting in 162 g, 175 g and 206 g of soil, respectively. In the field, two 55 cm-long core samples were taken at a distance of 40 cm from a virus-infected tree (320 g). 100 g of each sample was analysed for nematodes by the larvae migration procedure using a Baerman-funnel (Eppo, 2013), followed by microscopic identification of the nematode species.
Inoculation of donor trees. All samples from the experimental stock of ‘Bittenfelder’ seedlings examined before inoculation tested negative for the viruses investigated. A test of the chip-budded trees from experiment I and II about three months post inoculation revealed, that not all of the trees were successfully inoculated (Table 2). All replicates from experiment I tested positive in June 2013 for ACLSV and for ApMV also except for one tree. The same applied to experiment II. The ApMV-negative trees from both experiments were replaced the following spring by verified ACLSV/ApMV-positive trees from a stock of reserve trees (data not shown). At the first examination of ASGV/ASPV-inoculated plants of experiment I and II all trees were ASPV-positive but only one tree of each experiment was ASGV-positive. The re-inoculation of ASGV-negative trees in August 2013 was successful for a further two and three trees of experiment I and II in October, respectively. The trees that still tested negative in December 2013 were replaced by ASGV/ASPV-positive trees in April 2014. One tree infected with ASGV/ASPV was found dead in spring 2014 and was replaced in June 2014.
Table 2. Virus status of ‘Bittenfelder’ seedlings after inoculation with ACLSV/ApMV- or ASGV/ASPV-infected bark chips of experiment I and II.
| ACLSV/ApMV |
| ASGV / ASPV | |||||||
Sampling date | Jun 2013 |
| Jun 2013 | Oct 2013 | Dec 2013 |
| Jun 2013 | Oct 2013 | Dec 2013 | |
Experiment | I | II |
| Ib | I | I |
| IIb | II | II |
Pot N° |
|
|
|
|
|
|
|
|
|
|
1 | +/+ | +/+ |
| -/+ | +/+ | +/+ |
| -/+ | +/+ | +/+ |
2 | +/+ | +/+ |
| -/+ | -/+ | -/+ a |
| -/+ | -/+ | +/+ |
3 | +/– a | +/+ |
| -/+ | +/+ | -/+ c |
| -/+ | +/+ | -/+ |
4 | +/+ | +/– a |
| +/+ | +/+ | +/+ |
| -/+ | +/+ | +/+ |
5 | +/+ | + + |
| -/+ | -/+ | +/+ |
| +/+ | -/+ | +/+ |
6 | +/+ | +/+ |
| -/+ | -/+ | -/+ a |
| -/+ | -/+ | +/+ |
+ = virus-positive, - = virus-negative, a Replaced by either an ACLSV/ApMV or ASGV/ASPV-positive tree in April 2014. b Trees with negative RT-PCR result were inoculated a second time by bark chips from an ASGV-positive tree in August 2013. c Tree died and was replaced by an ASGC/ASPV-positive tree in June 2014. | ||||||||||
Experiment I. The first-time detection and the respective virus(es) found in recipient trees of experiment I is shown in Fig. 2. In pots with the virus combination ACLSV/ApMV five recipient trees were infected, two trees with both viruses and three trees with ACLSV only. The first transmission of ACLSV/ApMV was recorded 17.5 months after exposure. One ACLSV infection was identified 26 months after the trial started. All other infections were detected at the final testing. In pots with the virus combination ASGV/ASPV six recipient trees were infected, with two trees testing positive for both viruses and two trees each for ASGV and ASPV. The first transmission for ASPV and ASGV was recorded after 26 months in two plants of the same pot and after 36.5 months, respectively, after the trial started. At the end of the observation period a total of six plants were infected. For both virus combinations most new infections were recorded between the third and the fourth year of exposure. In total, virus transmissions were found in four of six pots for both virus combinations and 11 out of 24 (46%) recipient trees were infected. Single infections were recorded almost twice as often compared to double infections.
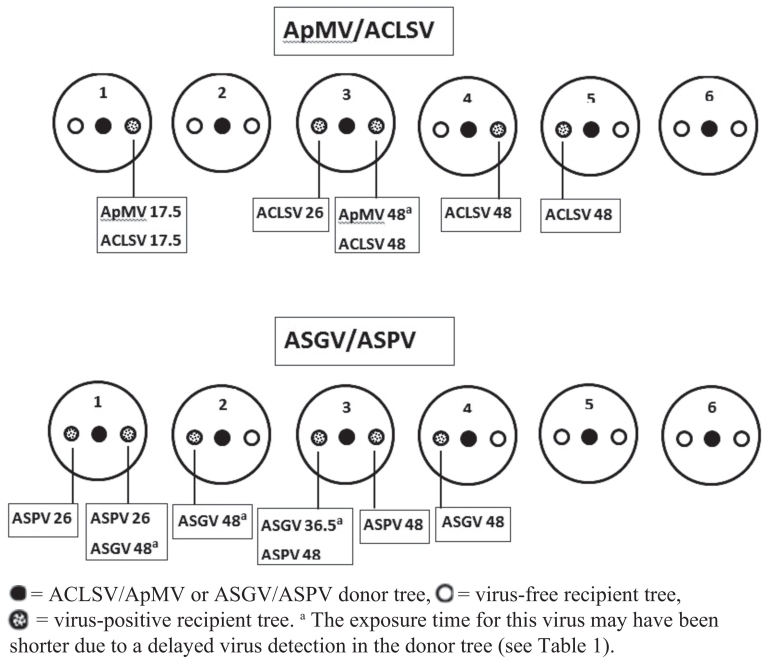
Fig. 2. Graphical summary of experiment I. Information on the months of the first virus detection post exposure and of the respective virus identified in recipient trees is given in boxes.
Experiment II. In experiment II only a single infection with ACLSV could be detected in one of the recipient trees 27 months post exposure (data not shown). The protective outer- and innermost nets were found free from ruptures by visual inspection. The microscopic examination of the 80 μm mesh net after 40 months of plant growth (October 2016) showed many thin root segments adhering to the net surface (Fig. 3A). Roots penetrating the net meshes had a diameter up to about 300 μm, forming root constrictions at the passageway (not shown). Meshes may have been stretched to some extent by this process. Net-crossing roots significantly smaller than 80 μm were also found (Fig. 3B), but it cannot be excluded that some of the roots belonged to weed plants which grew in the pots at times.
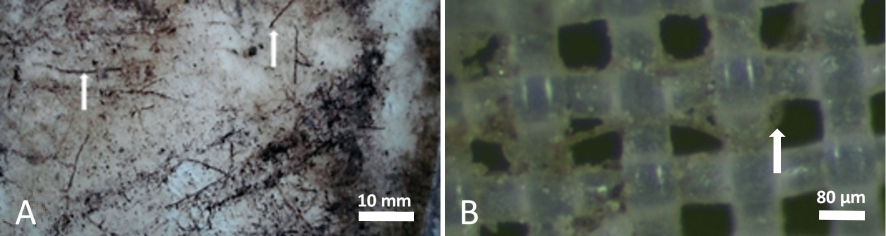
Fig. 3. A) Section (32 cm2) of the 80 μm mesh size polyamide net with adherent root segments. B) A root smaller than 80 μm in diameter crossing the mesh (magnification 100 x).
Experiment III. In experiment III leaf samples from recipient trees were examined 10, 15 and 21 months after irrigation with the eluate generated from experiment I. No virus-positive plants were detected. Likewise, none of the four viruses could be detected by RT-PCR in the soil eluates of experiment I or II.
The virus-inoculated trees in the field experiment were tested 14 weeks post inoculation by RT-PCR for the presence of viruses. All ACLSV/ApMV-inoculated donor trees were positive for both viruses. In the ASGV/ASPV set, all 10 trees tested positive for ASPV, but only two of them for ASGV (Table 3). A second bark chip inoculation with ASGV in August 2013 increased the number of ASGV-positive trees to seven when tested in October. Trees for which all three tests gave negative results for ASGV were replaced by ASGV/ASPV-positive plants the following spring (data not shown).
Table 3. Virus status of ‘Bittenfelder’ seedlings after inoculation by ACLSV/ApMV- or ASGV/ASPV-infected bark chips in the field trial.
| ACLSV |
| ApMV |
| ASGV |
| ASPV | ||
Sampling date | Jun 2013 |
| Jun 2013 a | Oct 2013 | Dec 2013 |
| Jun 2013 | ||
3 | + |
| + |
| - | - | + |
| + |
6 | + |
| + |
| - | - | - b |
| + |
9 | + |
| + |
| - | + | + |
| + |
12 | + |
| + |
| - | - | - b |
| + |
15 | + |
| + |
| - | + | + |
| + |
18 | + |
| + |
| + | + | - |
| + |
21 | + |
| + |
| + | + | + c |
| + |
24 | + |
| + |
| - | + | + |
| + |
27 | + |
| + |
| - | + | + |
| + |
30 | + |
| + |
| - | + | + |
| + |
+ = virus-positive, - = virus-negative. a All trees with negative RT-PCR result were inoculated a second time by bark chips from an ASGV-positive tree in August 2013. b Tree was replaced by a virus-positive tree in April 2014. c Tree found dead in June 2014 and was replaced by a virus-positive tree. | |||||||||
In row A a total of five out of 44 (11%) recipient trees tested virus-positive by the end of the experiment after 46 months (Fig. 4). In the ACLSV/ApMV block two trees were infected, one with both viruses and the other with ACLSV only. The infected recipient trees were neighbours of the same donor tree. In the ASGV/ASPV block three infected recipient trees were detected. Two trees were infected with both viruses and one tree with ASPV only.The earliest infection was detected in the ASGV/ASPV block 17.5 months after planting. The exposure time of the recipient trees for two of three ASGV transmissions may have been shorter due to the re-inoculation of donor trees (Fig. 4, lower panel No. 3 and 29). However, considering both blocks, the majority of virus infections were recorded at the end of the observation period, after 46 months. In the parallel downhill row (B) of recipient plants, only a single infection with ASGV was detected 36 months after planting.
The dimension of the root system of the unearthed ‘Bittenfelder’ seedlings showed a diameter of about 40 cm one year after planting (Fig. 5A). After 42 months the root system diameter extended to about 110–120 cm with the observation of multiple roots intertwining with those of the neighbouring tree (Fig. 5B). The complete dimensions, including the fine root system were not determined, but it obviously extended beyond the cleared-area in both cases.

Fig. 5. A) Dimension of the root system of ‘Bittenfelder’ seedling trees unearthed 12 months after planting. B) Superficially exposed root system 42 months after planting.
No plant parasitic or virus-transmitting nematodes (e.g. Eudorylaimus sp., Longidorus sp., Paratrichodorus sp., Trichodorus sp., Xiphinema sp.) were found in the soil samples of the three pots, where virus-infected recipient trees were identified until August 2016, nor in the samples around a virus-infected tree in the field trial taken at the same time.
In this research virus transmission between donor and recipient trees was studied in pot trials and in a field experiment. After four years almost half of the non-inoculated trees in pot experiment I and about one tenth of the non-inoculated trees in the field were infected by one or two viruses. In contrast to this, only a single transmission was observed each in pot experiment II, in which the root contact was limited, and in the parallel row of plants in the field.
Apple tree cultivars and rootstocks have been often reported to be naturally infected by multiple viruses (Wood, 1974; Kundu, 2003; Çağlayan et al., 2006) and therefore double-infected trees were used for the experiments. Comparing both the course of time and the scope of virus infections of recipient trees 18 months post exposure in pot experiment I and the field trial (row A), there was little difference in the transmission incidence. In each experiment only a single tree became double-infected. Assuming virus transmission by root contact, the conditions for root interlacing in both trials should be considered. In the pot experiment, donor and recipient trees grew close together, producing favourable conditions for early virus transmission. The root system of the trees overlapped to some extent at planting and a quick intertwining can be assumed. Therefore, virus transmission may have happened earlier than in the field trial but was recorded at the same time due to procedural planning. However, the first virus transmission in the field trial was expected to occur not sooner than 12 months post planting due to a lack of contacts demonstrated by the unearthed ‘Bittenfelder’ trees. The extent of the root system after one year of growth was unlikely to allow fine or finest root interlacing due to the space between neighbouring trees. While in the pot experiment further infections were detected after 26 and 36.5 months of exposure, this was not the case in the field trial. Considering the entire experimental period, a more even progression of virus transmission was expected due to increasing root intertwinings and root contacts between neighbouring trees. Increasing infections of adjacent trees by root grafting correlated with the progressing age and size of trees was demonstrated for the virus-caused psorosis disease on citrus (Bitancourt & Fawcett, 1944; cited in Epstein, 1978). The conditions under which root grafts are formed, however, are a subject of discussion. While some assume that radial growth and progressing pressure at contact points are a necessity (Graham & Bormann, 1966), others consider the tree age and root thickness as decisive (Lev-Yadun, 2011). Contrary to this Lešnik et al. (2008) concluded from experiments with apple proliferation-infected M9 rootstocks also that very tiny root bridges not visible to the naked eye are involved in the phytoplasma transmission. A possible lack of a “sufficient” root thickness for the formation of root grafts, as stated by Lev-Yadun (2011), might explain the low transmission rate in the pot and field trial within the first three years and the significant increase of infected recipient plants between the third and fourth year. The only infection detected in the parallel row B took a longer time of exposure than the earliest infection within row A, which would match to a much greater distance between virus donor and recipient trees. According to Epstein (1978), the density of the stand seems to be the most important prerequisite for grafting to occur as root grafting frequency increases with decreasing distance between trees. Moreover, roots may spread more within a row than between rows, as it was found by Henkel (2012) for an apple rootstock stool bed. So, the probability for root contacts and virus transmission between rows would be significantly lower than within the row.
Double infections by bark chip inoculation with ApMV or ASGV in combination with ACLSV or ASPV, respectively, could not be reliably obtained. The reasons may have been low virulence of the specific virus strain or an insufficient virus titre in the bark chips. The latter could apply particularly to ASGV, because low concentrations of this virus have been reported in the stem tissue of different apple cultivars, also in relation to other occurring apple viruses (Fuchs et al., 1988; Knapp et al., 1995; Paduch-Cichal & Tomala, 2007; Schröder, 2018). Regardless, in some cases where the inoculation resulted in delayed or negative virus detections and trees had to be re-inoculated or replaced, the real exposure time may have been shorter than for those trees that were virus-positive right from the beginning. This might have reduced the total number of observed transmissions.
Only a few detailed reports on the natural spread of the pome fruit viruses investigated in this study are available. Studies from New Zealand and India reported on the spread of ApMV from artificially infected apple seedlings to adjacent trees after four to six months. In those experiments the trees were only 25 cm apart or were planted a long time before inoculation and root grafts were assumed to have already existed at the time of inoculation (Anonymous, 1956; cited by Hunter et al., 1958; Dhingra, 1972). Therefore, the transmission rates of those studies cannot be compared with that of the current study. In a field trial of ACLSV transmission in apple McCrum (1965) reported similar periods of time for virus transmission as observed in this investigation, but unfortunately, the tree spacing was not mentioned. In the present study, the experimental conditions resulted in most virus transmissions between the third and fourth years of growth.
In pot experiment II, where the root contact between donor and recipient trees was restricted by polyamide nets, just a single virus transmission was recorded compared to the much higher transmission rate in pot experiment I, suggesting that the involvement of roots in virus transmission can be reasonably assumed. However, only roots smaller than about 300 μm were found passing the net barrier and therefore roots of this diameter seem to allow virus transmission, although at a much lower rate.
The transmission rate for an individual virus was inconsistent. Whereas ACLSV was transmitted about twice as often as ApMV in the pot I and the field trial, the transmission rate of ASGV and ASPV was identical. Moreover, the number of single and double infections of recipient trees was equal in the field, but not in the pot I experiment, where single infections were recorded almost twice as often compared to double infections. The reason for this is unknown as in case of a virus transmission via root grafting/anastomosis those differences would not be expected.
Although the presence of ACLSV and ASGV in root tips of infected apple trees has been shown by Kinard et al. (1996), the present study gave neither evidence for the release of viruses into the soil nor for the carrier function of soil water. Most of the plant viruses that have been detected in environmental waters so far belong to the genera of Carmo-, Cucumo-, Diantho-, Necro-, Potex-, Tobamo- and Tombusviruses and are mostly considered to be stable or extraordinary stable, but some viruses like Tobacco necrosis virus (TNV) or Cucumber mosaic virus (CMV) have been classified as rather unstable (Koenig, 1986; Mehle & Ravnikar, 2012; Mehle et al., 2018). Recently, a number of plant viruses from further genera were detected in wastewater (Bačnik et al., 2020). However, the pome fruit viruses examined in this study are not members of any of these genera. The survival of a virion in a changing external environment depends vitally on the stability of the capsid (Stone et al., 2019). Virion stability is usually estimated by the longevity of the sap infectivity in vitro (LIV). Comparing these data for the viruses examined in this study, ACLSV and ASGV can be rated as slightly more stable than ApMV and ASPV, but in relation to the majority of plant viruses found so far in aqueous environments, these viruses must be regarded as rather unstable (data on LIVs, as far as available, were taken from the database of Brunt et al. (1996) and onwards). However, virus stability in plant extracts does not correspond to that in the soil (Kegler et al., 1991). Stability, infectivity and adsorption of viruses in the soil environment or water is influenced by many factors like the pH-value, ion concentration, temperature, moisture, soil texture, organic matter content, the biological activity in the soil and virus specific properties, respectively (Kegler et al., 1991, 1995; Kimura et al., 2008; Xagoraraki et al., 2014; Mehle et al., 2018; Pinon & Vialette, 2018). Moreover, for some viruses it was demonstrated that infectivity depends on the concentration of the pathogen in water (Mehle et al., 2014). Assuming that in pot trial I and II virus release from roots took place, a very low concentration and/or a strong adsorption to soil components would explain why virions could not be detected in the soil washout or being able to infect successfully. The soil solution in the conducted field trial has not been examined for the presence of viruses and therefore this pathway cannot be excluded and may apply, in particular to the single transmission between row A and the downhill positioned row B.
In the present study no plant-parasitic nematodes were found in the soil samples from the pot- and field trial. Therefore there was no evidence that nematodes were implicated in virus transmission. Likewise, there was no indication for the involvement of alternative pathways of virus transmission mentioned in the introduction. To my knowledge, none of these transmission routes have been verified in the literature for any of the pome fruit viruses on apple examined here.
Projecting the experimental pot and field results of this study to the situation in nurseries or scion producing orchards, soil transmission of the four pome fruit viruses may become a serious problem after a few years of closely spaced tree growth. Particularly the conditions in the pot experiment I revealed a high potential of virus spread and may resemble the situation of vegetatively propagated apple rootstocks along a row in stool bedding production. Although root grafting was not directly examined in the present study, the circumstantial evidence strongly suggests this pathway. Virus spread by soil transmission can be rated as rather slow compared to insect transmission. However, due to the latency of most of the examined viruses natural spread could go unnoticed for years and the risk of economic impacts might therefore be underestimated.
This study (project number 2812NA049) was part of a joint research project with the Federal Research Centre for Cultivated Plants, Institute for Plant Protection in Fruit Crops and Viticulture in Dossenheim, funded by the Federal Ministry for Food and Agriculture in frame of the federal program for ecological land use and other forms of sustainable agriculture. The responsibility for the contents of this publication lies with the author. The author would like to thank Sandra Brox-Viehmann for excellent technical assistance, Dr. Carolin Zimmermann for analysing a part of the plant samples, Dr. Olaf Zimmermann for soil investigation on nematodes and Dr. Klaus Michels for soil analysis. Constanze Berwarth from the Institute for Plant Protection in Fruit Crops and Viticulture in Dossenheim will be thanked for inoculation of the experimental trees with virus isolates and Hermann Meschenmoser (LTZ) and Wolfgang Bauer from the office for agriculture in Bruchsal are acknowledged for technical support in carrying out the pot and field trials. Dr. Bernd Schneider is very much thanked for performing the ultracentrifugation of the soil eluates and critical reviewing the manuscript and language improvement.
The author declares that he has no competing interests.
Akbaş, B.¸ K. Değirmenci, 2009: Incidence and natural spread of Apple mosaic virus on hazelnut in the West Black Sea Coast of Turkey and its effect of yield. Journal of Plant Pathology 91 (3), 767-771.
Anonymous, 1991: Das VDLUFA Methodenbuch Band 1: Die Untersuchung von Böden. VDLUFA-Verlag Darmstadt.
Anonymous, 2014: Commission implementing directive 2014/98/EU, implementing Council Directive 2008/90/EC as regards specific requirements for the genus and species of fruit plants referred to in Annex I thereto, specific requirements to be met by suppliers and detailed rules concerning official inspections, Official Journal of the European Union L 298/22, amended by Commission implementing directive (EU) 2020/177, Official Journal of the European Union L 41/1.
Arli Sökmen, M., N.D. Kutluk Yilmaz, H. Mennan, M.A. Sevik, 2005: Natural weed hosts of Apple mosaic virus in hazelnut orchards in Turkey. Journal of Plant Pathology 87 (3), 239-242.
Bačnik, K., D. Kutnjak, A. Pecman, N. Mehle, M. Tušek Žnidarič, I. Gutiérrez Aguirre, M. Ravnikar, 2020: Viromics and infectivity analysis reveal the release of infective plant viruses from wastewater into the environment. Water Research 177, p. 115628, DOI: 10.1016/j.watres.2020.115628.
Brunt, A.A., K.Crabtree, M.J. Dallwitz, A.J.Gibbs, L.Watson, E.J. Zurcher, (Eds.), 1996, Plant Viruses Online: Descriptions and Lists from the VIDE Database. Version: 20th August 1996: URL: http://bio-mirror.im.ac.cn/mirrors/pvo/vide/refs.htm, Access: 20th January 2021.
Çağlayan, K., Ç. Ulubaş SerÇe, M. Gazel, W. Jelkmann, 2006: Detection of four apple viruses by ELISA and RT-PCR assays in Turkey. Turkish Journal of Agriculture and Forestry 30, 241-246.
Ciccotti, A.M., P.L. Bianchedi, P. Bragagna, M. Deromedi, M. Filippi, F. Forno, L. Mattedi, 2007: Transmission of ‘Candidatus Phytoplasma mali’ by root bridges under natural and experimental conditions. Bulletin of Insectology 60 (2), 387-388.
Dhingra, K.L., 1972: Transmission of Apple mosaic by natural root grafting. Indian Journal of Horticulture 29, 348-350.
Eppo, 2013: PM 7/119 (1) Nematode extraction. Bulletin OEPP/EPPO Bulletin 43 (3), 471-495.
Epstein, A.H., 1978: Root graft transmission of tree pathogens. Annual Review of Phytopathology 16, 181-192.
Fritzsche, R., H. Kegler, 1968: Nematoden als Vektoren von Viruskrankheiten der Obstgewächse. Tagungsberichte Deutsche Akademie der Landwirtschaftswissenschaften Berlin 97, 289-295.
Fuchs, E., M. Grüntzig, B. Al Kai, 1988: Der serologische Nachweis mechanisch übertragbarer Viren des Kern- und Steinobstes. Nachrichtenblatt für den Pflanzenschutz in der DDR 42 (10), 208-211.
Golino, D.A., S.T. Sim, M. Cunningham, A. Rowhani, 2007: Transmission of rose mosaic viruses. Acta Horticulturae 751, 217-224, DOI: 10.17660/ActaHortic.2007.751.26.
Graham, B.F., Jr., F.H. Bormann, 1966: Natural root grafts. The Botanical Review 32, 255-292.
Grimová, L., L. Winkowska, M. Konradi, P. Ryšánek, 2016: Apple mosaic virus. Phytopathologia Mediterranea 55, 1-19. DOI: 10.14601/Phytopathol_Mediterr-16295.
Henkel, G., 2012: Bei Wurzelverwachsungen ist das Risiko gering. Deutsche Baumschule issue 2, 36-38.
Hunter, J.A., E.E. Chamberlain, J.D. Atkinson, 1958: Note on the transmission of Apple mosaic by natural root grafting. New Zealand Journal of Agricultural Research 1, 80-82.
Jelkmann, W., S. Paunovic, 2011: Apple stem pitting virus, p. 35-40, in: Virus and Virus-like diseases of pome and stone fruits. A. Hadidi, M. Barba, T. Candresse, W. Jelkmann (Eds.), APS Press, St. Paul, Minnesota.
Kegler, G., H. Kleinhempel, H. Kegler, 1980: Untersuchung zur Bodenbürtigkeit des tomato bushy stunt virus. Archiv für Phytopathologie und Pflanzenschutz 16 (2), 73-76.
Kegler, H., E. Fuchs, D. Spaar, J. Kegler, 1995: Viren im Boden und Grundwasser (Übersichtsbeitrag). Archives of Phytopathology and Plant Protection 29 (4), 349-371.
Kegler, H., H-G. Kontzog, H. Kleinhempel, 1991: Das Verhalten pflanzenpathogener Viren im Boden (Übersichtsarbeit). Archiv für Phytopathologie und Pflanzenschutz, Berlin 27 (5), 339-346.
Kimura, M., Z.J. Jia, N. Nakayama, S. Asakawa, 2008: Ecology of viruses in soils: Past, present and future perspectives. Soil Science and Plant Nutrition 54, 1–32, DOI: 10.1111/j.1747-0765.2007.00197.x.
Kinard, G.R., S.W. Scott, O.W. Barnett, 1996: Detection of Apple chlorotic leaf spot and Apple stem grooving viruses using RT-PCR. Plant Disease 80, 616-621.
Knapp, E., A. da Câmara Machado, H. Pühringer, Q. Wang, V. Hanzer, H. Weiss, B. Weiss, H. Katinger, M. Laimer da Câmara Machado, 1995: Localization of fruit tree viruses by immuno-tissue printing in infected shoots of Malus sp. and Prunus sp. Journal of Virological Methods 55, 157-173.
Koenig, R., 1986: Plant viruses in rivers and lakes. Advances in Virus Research 31, 321-333.
Kundu, J.K., 2003: The occurrence of Apple stem pitting virus and Apple stem grooving virus within field-grown apple cultivars evaluated by RT-PCR. Plant Protection Science 39 (3), 88-92.
Lešnik, M., J. Brzin, N. Mehle, M. Ravnikar, 2008: Transmission of ‚Candidatus phytoplasma mali‘ by natural formation of root bridges in M9 apple rootstock. Agricultura 5, 43-46.
Lev-Yadun, S., 2011: Why should trees have natural root grafts? Tree Physiology 31, 575–578, DOI: 10.1093/treephys/tpr061.
Massart, S., M.H. Jijakli, J. Kummert, 2011: Apple stem grooving virus, p. 29-33, in: Virus and Virus-like diseases of pome and stone fruits. A. Hadidi, M. Barba, T. Candresse, W. Jelkmann (Eds.), APS Press, St. Paul, Minnesota.
McCrum, R.C., 1965: Spread of apple chlorotic leaf spot virus from tree to tree. Plant Disease Reporter 49, 958-959.
Mehle, N., I. Gutiérrez-Aguirre, D. Kutnjak, M. Ravnikar, 2018: Water-mediated transmission of plant, animal, and human viruses. Advances in Virus Research 101, 85-128, DOI: 10.1016/bs.aivir.2018.02.004.
Mehle, N., I. Gutiérrez-Aguirre, N. Prezelj, D. Delić, U. Vidic, M. Ravnikar, 2014: Survival and transmission of Potato Virus Y, Pepino Mosaic Virus, and Potato Spindle Tuber Viroid in water. Applied and Environmental Microbiology 80, 1455-1462, DOI: 10.1128/AEM.03349-13.
Mehle, N., M. Ravnikar, 2012: Plant viruses in aqueous environment – Survival, water mediated transmission and detection. Water Research 46, 4902-4917, DOI: 10.1016/j.watres.2012.07.027.
Menzel, W., W. Jelkmann, E. Maiss, 2002: Detection of four apple viruses by multiplex RT-PCR assays with coamplification of plant mRNA as internal control. Journal of Virological Methods 99, 81-92.
Paduch-Cichal, E., K. Tomala, 2007: Detection of Apple chlorotic leaf spot virus (ACLSV) and Apple stem grooving virus (ASGV) in different tissues of ‘Mutsu’ apple cultivar trees by ELISA. Phytopathologia Polonica 43, 53-59.
Pethybridge, S.J., C.R. Wilson, F.S. Hay, G.W. Leggett, L.J. Sherriff, 2002: Mechanical transmission of Apple mosaic virus in Australian hop (Humulus lupulus) gardens. Annals of Applied Biology 141, 77-85.
Petrzik, K., O. Lenz, 2011: Apple mosaic virus in Pome Fruits, p. 25-28, in: Virus and Virus-like diseases of pome and stone fruits. A. Hadidi, M. Barba, T. Candresse, W. Jelkmann (Eds.), APS Press, St. Paul, Minnesota.
Pinon, A., M. Vialette, 2018: Survival of viruses in water. Intervirology 61, 214-222, DOI: 10.1159/000484899.
Roberts, A.G., 2014: Plant viruses: Soil borne. In: eLS. John Wiley & Sons, Ltd: Chichester. DOI: 10.1002/9780470015902.a0000761.pub3.
Schröder, M., 2018: Seasonal detection of pome fruit viruses by RT-PCR in apple mother trees used for scion production. Journal für Kulturpflanzen 70, 124-129, DOI: 10.1399/JKI.2018.04.02.
Sevik, M.A., 2011: Water pollution: Water-borne plant viruses. Erciyes Üniversitesi Fen Bilimleri Enstitüsü Dergisi 27 (1), 40-47.
Smith, P.R., R.N. Campbell, P.R. Fry, 1969: Root discharge and soil survival of viruses. Phytopathology 59, 1678-1687.
Stone, N.P., G. Demo, E. Agnello, B.A. Kelch, 2019: Principles for enhancing virus capsid capacity and stability from a thermophilic virus capsid structure. Nature Communications 10, 4471, DOI: 10.1038/s41467-019-12341-z.
Teakle, D.S., 1986: Abiotic transmission of Southern bean mosaic virus in soil. Australian Journal of Biological Sciences 39, 353-359.
Wood, G.A., 1974: Latent viruses of apple in New Zealand. New Zealand Journal of Agricultural Research 17 (1), 85-91, DOI: 10.1080/00288233.1974.10421085.
Xagoraraki, I., Z. Yin, Z. Svambayev, 2014: Fate of viruses in water systems. Journal of Environmental Engineering, 04014020, 1–18, DOI: 10.1061/(ASCE)EE.1943-7870.0000827.
Yaegashi, H., N. Yoshikawa, T. Candresse, 2011: Apple chlorotic leaf spot virus in pome fruits, p. 17-21, in: Virus and Virus-like diseases of pome and stone fruits. A. Hadidi, M. Barba, T. Candresse, W. Jelkmann (Eds.), APS Press, St. Paul, Minnesota.
Zhang, L., W. Jelkmann, 2017: Construction of full-length infectious cDNA clones of Apple chlorotic leaf spot virus and their agroinoculation to woody plants by a novel method of vacuum infiltration. Plant Disease 101 (12), 2110-2115, DOI: 10.1094/PDIS-04-17-0573-RE.