

Journal für Kulturpflanzen, 74 (03-04). S. 85–93, 2022 | DOI: 10.5073/JfK.2022.03-04.06 | Becker et al.
Bacteria producing contractile phage tail-like particles (CPTPs) are promising alternatives to conventional pesticides
Bakterien, die kontraktile Phagenderivate produzieren, sind vielversprechende Alternativen zu konventionellen Pflanzenschutzmitteln
 | (c) The author(s) 2022 This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/deed.en). |
Submitted/accepted for publication: 20 December 2021/1 March 2022 |
This mini-review aims at raising the interest in contractile phage tail-like particles (CPTPs) of bacteria as an efficient and pest-specific alternative to conventional chemical pesticides in agriculture, horticulture and forestry. CPTPs are used by various bacteria in diverse environments for interbacterial competition or for manipulation of eukaryotic hosts, such as fungi or insects. This review gives examples for the versatile use of CPTPs as powerful biological control agents. We introduce the different types of CPTPs with a special focus on those with activity against insect plant pests. In addition, we present two currently established web services that combine the permanently increasing knowledge on CPTPs with a selection approach of the best candidate bacteria for targeted CPTP application in sustainable plant production.
bacteria, bacteriophages, contractile phage tail-like particles, pest control, insecticides
Diese Übersichtsarbeit verfolgt das Ziel, über bakterielle kontraktile Phagenderivate (englisch CPTPs) und ihr Potenzial als effiziente und Schadorganismus-spezifische Alternativen zu konventionellen chemischen Pflanzenschutzmitteln in der Land-/Forstwirtschaft und im Gartenbau zu informieren. CPTPs werden von verschiedenen Bakterien in diversen Habitaten für den interbakteriellen Konkurrenzkampf sowie zur Beeinflussung eukaryotischer Wirte, wie Pilze und Insekten, verwendet. Diese Arbeit präsentiert interessante und bemerkenswerte Beispiele für den vielfältigen Einsatz von CPTPs als leistungsfähige biologische Bekämpfungsmittel. Wir stellen die verschiedenen Typen von CPTPs vor und legen einen besonderen Fokus auf diejenigen, die eine Wirkung gegen Schadinsekten besitzen. Zusätzlich stellen wir zwei kürzlich etablierte Webservices vor, die das permanent wachsende Wissen über CPTPs mit einem Auswahlverfahren für die besten Bakterienkandidaten kombinieren, um eine zielgerichtete Anwendung der CPTPs in der nachhaltigen Pflanzenproduktion zu ermöglichen.
Bakterien, Bakteriophagen, kontraktile Phagenderivate, Pflanzenschutz, Insektizide
Plant pests are a thread to plant production and forestry. Loss of biodiversity and climate change create new ecological niches, which can be easily occupied by invasive species, such as plant pathogenic bacteria, fungi, nematodes, and insects. Phytosanitary measures for eradication of quarantine pests or – once stable populations have established – the containment of invasive pests, is expensive. Moreover, one needs to mention the costs of harvest losses caused by pests that developed resistance to conventional chemical pesticides and those that developed mechanisms to break crop resistance. Environment-friendly crop production and forestry rely on breeding of resilient varieties, habitat biodiversity and sustainable farm/forest management practices. Recently the potential of in situ plant-associated microorganisms, such as bacteria and fungi, has gained high awareness, because of their plant-beneficial capabilities, e.g. to reduce the deleterious impact of pests by enhancing plant resilience or by direct pest-antagonizing mechanisms. Targeted application of these microorganisms is used to enhance plant performance and/or support the native (micro-) biota of plants in (agro/forest-) ecosystems (Glick, 2012; Kondo et al., 2020; Lopes et al., 2021).
Here, we present an alternative plant protection strategy that addresses pests individually. The approach is based on the application of bacteria that naturally produce derivatives of bacterial viruses (bacteriophages or prophages, short: phages), that lost their viral autonomy and have been transformed over a long evolutionary time into tools for interbacterial competition and interaction with eukaryotes, called “phage tail-like particles” (CPTPs). Several types of phage derivatives differing in their mode of action have been described, and different synonyms for the same type of phage derivative have been used in recent publications. Previously, we have described the existing synonyms in more detail (Patz et al., 2019) and seek to simplify the (sometimes misleading) terminology for this mini-review. CPTP types, subgroups, and most frequently used synonyms are presented in Table 1.
Table 1. Contractile phage tail-like particles (CPTPs): Main types, frequently used synonyms, and subgroups (for more detail see Patz et al., 2019)
Type | (Rigid contractile) Tailocin | Extracellular Contractile Injection System: eCIS | Type VI Secretion System: T6SS |
synonyms | Rigid-Type Tailocin R-Type Tailocin R-Type Pyocin Pyocin | Toxin Delivery Tailocin PLTS (Phage-like Protein Translocation Structure) | no synonyms |
subgroups | no subgroups | Afp, AfpX (Antifeeding prophage, tailocin designated Afp) PVC (Photorhabdus Virulence Cassette) MAC (Metamorphosis-associated contractile structure) BIS (Bacteroidales injection system) T6SS type IV | T6SS type I T6SS type II T6SS type III |
The idea to apply such phage derivatives to plants differs significantly from phage therapy, which has been suggested as part of an integrated plant disease management strategy (Balogh et al., 2010; Buttimer et al., 2017). In contrast to phages, phage derivatives do not need to be isolated from bacteria before application, do not require protective formulations or sheltering from sunlight, have a much higher persistence in the plant environment and can be used as part of an inoculum of CPTP-producing bacteria, providing the important benefit of plant-colonizing bacteria serving as vectors for phage derivatives. Most importantly, phages do only address bacteria, but phage derivatives, can affect different kinds of organisms, including insects.
However, the great potential of CPTPs in plant protection has been underestimated and research towards application in crop production and forestry has rarely been tackled until now (Lavermicocca et al., 2002; Príncipe et al., 2018). The different CPTP-types and their potential as alternatives to conventional chemical pesticides are presented in the course of this mini-review.
The term “phage tail-like” correctly suggests ancestral and structural similarities to bacteriophages, which are viruses of bacteria. However, in contrast to phages, CPTPs are not viruses: The CPTP syntenic gene clusters lack genes for viral DNA transfer, and for the capsid biosynthesis, acting as the viral DNA storage structure (Figure 1B). The latter was repeatedly confirmed microscopically and genetically (Fernandez et al., 2017; Patz et al., 2019). First-grade research on CPTPs has been published in the past few years unravelling the atomic structure (Ge et al., 2015; Chang et al., 2017; Jiang et al., 2019) and their fascinating mode of action (Taylor et al., 2016; Böck et al., 2017; Vacheron et al., 2021). Two characteristics describing their place of action (intracellular/membrane-anchored vs. extracellular space) and the mode of action (mechanical vs. effector/toxin delivery) allow differentiation of three main types of CPTPs, which are relevant for pest control: (i) type VI secretion systems (T6SSs) constituting the intracellular/membrane-anchored CPTPs, (ii) tailocins constituting mechanically functioning extracellular CPTPs and (iii) extracellular contractile injection systems (eCISs) constituting effector/toxin delivering extracellular CPTPs (Fig. 1A).
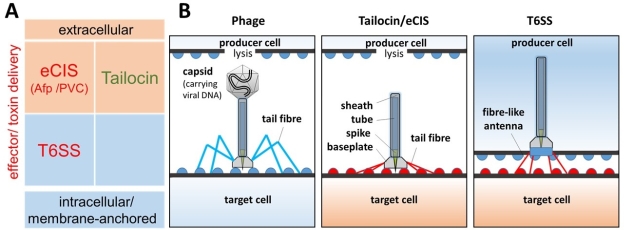
Fig. 1. Subgroups of contractile phage tail-like particles (CPTPs): A. Main types of CPTPs distinguished by two characteristics: (i) place of action: extracellular vs. intracellular/membrane-anchored, (ii) mode of action: effector/toxin delivery (in red font) vs. mechanical (in green font); B. Main structural differences between CPTPs and bacteriophages. Note that the target cell of the phage is from the same bacterial strain as the producer cell and becomes a putative new producer cell after infection, whereas target cells of tailocins, eCISs and T6SSs belong to a different bacterial strain and are supposed to be killed immediately. Half circles on the outside of bacterial cells represent specific surface determinants (e.g. lipopolysaccharides), which are recognized by tail fibres and fibre-like antennae, respectively. Created with Power Point.
Effector and toxin delivery CPTPs are of particular interest, since their mode of action includes injection of compounds into the target-organisms. Two types of effector/toxin delivery CPTPs have been distinguished: the first type (eCIS) functions outside the producer cells (Fig. 1 and 2). The extracellular activity by itself is not astonishing as many bacterial compounds are transported to the extracellular space via a large assortment of bacterial secretion systems, but eCISs are that large in size that it is impossible to transport them through the cell wall of a living cell. Thus, producer cells have to lyse (and die) in order to release eCISs. In other words, producer cells “sacrifice” themselves for the sake of the population. From a human point of view, this may appear altruistic, however, from an evolutionary point of view, “division of labour” (including death of particular cells) is a highly beneficial trait for the population. Well analysed eCISs (Afp, AfpX, PVC, MAC) target eukaryotic cells, however recent bioinformatic studies indicate that certain types of eCIS can target prokaryotes (Fig. 3), which needs to be confirmed experimentally (Geller et al., 2021). The second type of effector/toxin delivery CPTPs is the T6SS, which remains anchored inside the producer cell while being in action and does not require cell lysis (Fig. 1 and 2). Activity of T6SS against gram-negative bacteria and eukaryotic cells (reviewed in Monjarás Feria & Valvano, 2020) is well established. Recently activity of T6SS against yeast (Trunk et al., 2018) and gram-positive bacteria (Le et al., 2021) was shown as well.
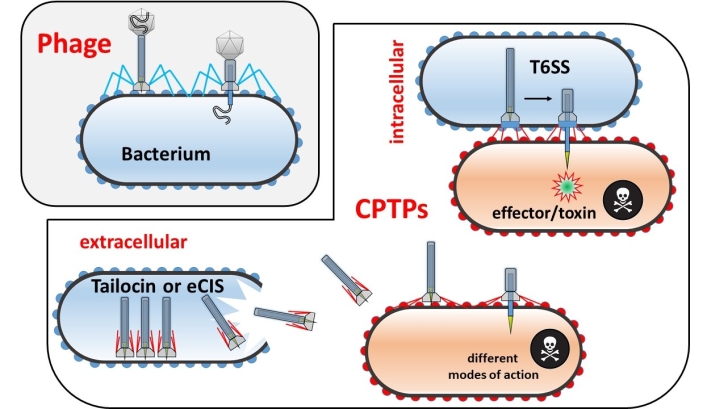
Fig. 2. Schematic depiction of morphological differences between a contractile phage, carrying a capsid with viral DNA, and contractile phage tail-like particles (CPTPs) lacking the capsid; the type VI secretion system (T6SS) differs from the extracellular CPTPs in its intracellular/membrane-anchored place of action. In contrast to the T6SS, there are multiple copies of an extracellular contractile injection system (eCIS) or a tailocin within a single bacterial cell. For simplification, the different modes of action of eCISs and tailocin are not depicted here, but in Fig. 3. Created with Power Point.
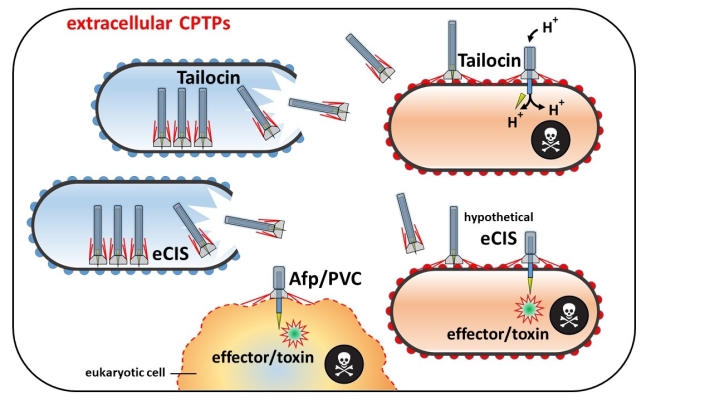
Fig. 3. Schematic depiction of functional differences between three types of extracellular CPTPs, causing death of the target cell: a tailocin functioning mechanically and two subgroups of extracellular contractile injection systems (eCISs) delivering effectors/toxins into target cells: (i) Antifeeding prophages (Afps) and Photorhabdus virulence cassettes (PVCs) attack insect (eukaryotic) cells, (ii) Bioinformatic studies indicate that certain types of (hypothetical) eCISs might target prokaryotes. Created with Power Point.
R-Type tailocins (rigid contractile tailocins) represent another important type of extracellular CPTPs, but their mode of action is different. Whereas eCIS and T6SS inject chemical compounds (toxins, effectors) into target cells, tailocins puncture a hole in the bacterial cell wall and the plasma membrane of the target, which causes loss of the proton motive force and subsequent starvation of the target cell (Fig. 3). Due to the homology between eCIS and R-Type tailocin syntenic gene clusters, the differentiation of both on a genomic scale is quite challenging and relies often on specific domains and the absence/presence of an effector/toxin-encoding gene close to the respective gene cluster. From an evolutional point of view, R-Type tailocins originates from the contractile phage family Myoviridae. However, we like to mention here, that there is another type of tailocins diverged from the family Siphoviridae that is non-contractile and termed F-Type (flexible) tailocin. Its potential is less documented and will not be further considered in this review. Both types of tailocins are well studied in gram-negative bacteria (reviewed in Ghequire & Mot, 2015 and Patz et al., 2019) but have also been identified in gram-positive bacteria (Gebhart et al., 2015; Lee et al., 2016).
All three types of CPTPs share the same physical composition. They are syringe-like devices or spear-like projectiles that consist of an outer contractile sheath and an inner rigid tube, a baseplate, which allows a tight binding to the target cell surface, and six tail fibres carrying receptor-binding proteins (RBPs) that guarantee specificity (Figure 3). However, the different places of action (inside or outside the producer cell) require substantial adaptations in the different types of CPTPs.
While almost all CPTPs are released from producer cells by cell lysis, the T6SS is attached to the cell envelope of its producer cell by a membrane anchor. The energy stored in the extended sheath is used to thrust the spike and tube with the associated effectors out of the producer cell and across membranes of both producer and target cell (Fig. 2). During the assembly of the T6SS, effectors or toxins are preloaded onto the spike or into the tube, and after action, subunits of the contracted sheath are recycled by a T6SS-specific unfoldase, to allow for a new round of assembly (Basler, 2015). At first glance, the recycling of the T6SS appears a much more efficient strategy than lysing the producer cell, but the numbers of active CPTPs per bacterial cell differ conspicuously. Usually, only a single T6SS per cell can be built at a time, but hundreds of extracellular CPTPs. Yao and co-workers calculated that Burkholderia cenocepacia releases on average approximately 600 tailocins from a lysed cell (Yao et al., 2017). Although T6SSs are believed to play a predominant role in bacteria-bacteria competition (Ho et al., 2014; Chen et al., 2015), recently also anti-yeast action has been demonstrated for Serratia marcescens against Saccharomyces cerevisiae and Candida albicans (Trunk et al., 2018). Trunk and co-workers showed that bacteria-yeast cell contact is essential for T6SS activity causing arrest of yeast cell growth and death. T6SS toxin delivering activity against filamentous fungi has not been shown yet, to the best of our knowledge. However this area of study could open up exciting new areas of bacterial-fungal interaction (BFI) research (Deveau et al., 2018) and potentially for biological control of phytopathogenic fungi by plant-associated bacteria. Tailocins are produced by bacteria to target closely related bacterial strains in the same biological niche (Dorosky et al., 2017; Scholl, 2017; Vacheron et al., 2021), but the target range might be extended by e.g. horizontal transfer of tail fibre genes or a higher mutation rate in the genetic domains of tail fibres (Haggård-Ljungquist et al., 1992; Yao et al., 2017).
Brackmann and co-workers (Brackmann et al., 2017) stated that using physical force to translocate macromolecules across a membrane, which requires a sharp tip, a source of energy, and the ability to strongly bind to the target, has the advantage of being a universal solution independent of the properties of the target membrane and cell wall. Regardless of this, specificity is provided by the target surface: All CPTPs, targeting either gram-negative bacteria, gram-positive bacteria or eukaryotic cells, have in common that target specificity is presumably conferred by tail fibre/receptor recognition. For instance, the properties of lipopolysaccharides (LPSs) on the outside of the target cell wall of gram-negative bacteria are crucial for a strong binding of CPTP tail-fibres. The density and specificity of LPS moieties, particularly O-specific antigens, renders them either a receptor for, or a shield against CPTPs and determines whether a strain is sensitive or resistant (Carim et al., 2021). Altered LPS composition is supposedly the reason why tailocin-producing strains manage to avoid self-intoxication. This has also been shown for T6SSs of Vibrio fischeri (Speare et al., 2021). LPS thinning seems to be a mechanism by which resistant strains can become more sensitive to tailocins (Carim et al., 2021). Gram-positive bacteria have no LPS on their outer surface, but carry peptidoglycans that may serve as receptors; however, this has to our knowledge, not been studied yet. Recently, it was speculated by Smith and co-workers that a single T6SS, though dependent on cell-cell contact, may have a broad target spectrum without relying on target cells’ surface receptors (Smith et al., 2020). Different from tailocins and eCISs cell-cell contact can be possibly established between T6SS-producing strains by other modes than tail fibre-binding. However, it was shown that T6SSs of Myxococcus xanthus (Chang et al., 2017) produce fibre-like antennae on the surface (also depicted in Fig. 1B), which the authors supposed to be important for recognizing targets.
Insecticidal activity was reported for T6SSs and two of the eCIS subgroups: Afps and PVCs. T6SSs have an impact on bacteria by using anti-prokaryotic effectors and an impact on yeast, insects and other animals using anti-eukaryotic effectors (Monjarás Feria & Valvano, 2020). Afps and PVCs attack exclusively insect cells. Further subgroups of eCIS (Table 1) have been described (Penz et al., 2012; Shikuma et al., 2014; Rojas et al., 2020), but have little or no insecticidal activity and thus are beyond the scope of this article. Afps, PVCs and T6SSs are applied by entomopathogenic bacteria to eventually invade the insect haemocoel. The haemocoel is the body cavity of insects filled by haemolymph, the insect “blood”, a fluid plasma that contains haemocytes, which are immune cells analogous to human leucocytes. Insect haemocytes bind to invading microorganisms and mediate immune responses like phagocytosis or encapsulation (Lavine & Strand, 2002). In the following, we present for each of the insecticidal CPTP subgroups one compelling example from literature, showing that CPTP-producing bacteria manage to invade the insect haemocoel, which appears to be an obligatory precondition for deploying a lethal effect.
T6SS: Vacheron and co-workers found that the T6SS of the plant-colonizing bacterium Pseudomonas protegens contributes significantly to insect pathogenicity in oral infection assays on larvae of the cabbage pest Pieris brassicae, while causing severe changes in the insect gut microbiome, but not when bacteria were injected directly into the haemocoel (Vacheron et al., 2019). The authors concluded that entomopathogenic bacteria deploy T6SS-based strategies to first disrupt the commensal microbiota and establish in the insect gut, before they pass through the peritrophic membrane and gut epithelial barrier, and proliferate in the haemocoel where they kill the host eventually by expression of virulence factors. In order to escape from haemocyte-mediated immunity of insects, entomopathogenic bacteria have evolved a highly protective cell envelope (Kupferschmied et al., 2016). Like P. protegens many other plant-colonizing bacteria have been reported to contain gene clusters encoding type VI secretion systems (Becker et al., 2018), but whether these T6SSs address prokaryotes or eukaryotes still needs to be elucidated, offering a giant pool of yet unused resources for “biological pesticides”.
Afp: The Serratia entomophila antifeeding prophage (Afp), located on a plasmid, causes amber disease in the New Zealand grass grub, Costelytra zealandica. The term “antifeeding” describes adequately the response, the cessation of feeding, of insect larvae two days after ingesting plant tissue colonized by Afp-producing bacteria. The name-giving amber colour of larvae appears one day later due to clearing of the usually dark larval gut. Infected larvae may remain in a chronic, non-feeding state for more than 4 months before bacteria eventually invade the haemocoel, resulting in rapid death of the insect (Jackson et al., 2001). Hurst and co-workers showed that the purified Afp caused the same symptoms in grass grub larvae and thus verified that Afp is the causative agent of amber disease (Hurst et al., 2007). Another species from genus Serratia, S. proteamaculans was also shown to produce antifeeding prophages, here termed AfpXs, targeting other New Zealand pasture pests: Grass Grub (Costelytra giveni) and Manuka Beetle (Pyronota species). Demonstrating that Serratia entomophila and S. proteamaculans have no impact on other Scarabaeidae insect species, the authors inferred a high target-specificity of Afps. Genome analyses revealed that other bacteria, such as the plant endophyte Erwinia oleae, contain Afp variants (Hurst et al., 2018) pointing to the unemployed potential of Afps to defeat insect pests other than those endemic to New Zealand.
PVC: Other CPTPs, which are very similar to Afps, have been discovered in bacteria from genera Xenorhabdus and Photorhabdus, which are mutualistically associated with entomopathogenic nematodes: Photorhabdus with nematodes from the family Heterorhabditidae and Xenorhabdus with family Steinernematidae (Hinchliffe, 2013). The bacteria use the nematodes as a vector to enter an insect, where they invade the haemolymph and produce toxins, which eventually kill the insect. A single strain of Xenorhabdus may produce a variety of bioactive compounds against bacteria, fungi, insects, nematodes, protozoa, and cancer cells (reviewed in Dreyer et al., 2018). Surprisingly, the PVC products of Photorhabdus have no antibacterial activity but trigger rapid destruction of insect haemocytes. Photorhabdus Virulence Cassettes inject insecticidal toxins directly into insect cells to cause actin cytoskeleton condensation (Yang et al., 2006). Produced for killing the blood cells of insects, the use of PVCs appears an extraordinary aggressive, though sophisticated bacterial strategy for escaping haemocyte recognition. Since the PVC products of Photorhabdus lack the antibacterial activity of T6SSs and tailocins, but possess a variable number of putative anti-insect effectors instead, Yang and co-workers hypothesized that the PVC products of Photorhabdus have been modified to attack eukaryotic host cells. Bacteria from both genera, Xenorhabdus and Photorhabdus, produce a multitude of insecticidal and other toxins. For Photorhabdus the significant impact of PVCs was shown, for Xenorhabdus this still needs to be confirmed, but genomic analyses revealed that Xenorhabdus strains also possess PVC gene clusters (Vlisidou et al., 2019).
Contractile tailocin: R-Type tailocins are the only CPTPs working mechanically and are effective bacteriocins/bactericides (Patz et al., 2019), nonetheless, a significant impact on insect pests has to the best of our knowledge not been shown. It is conceivable that R-Type tailocin-producing bacteria have the potential to affect significantly the gut microbiome of insects. However, the status quo of current CPTP research suggests that bacteria have to invade the insect haemocoel and secrete effectors for having a lethal effect on the insect.
Pollinators are in major focus when it comes to application of new plant protection products, and the application of potentially harmful compounds needs to be reduced. Thus, specificity of CPTPs needs to be verified to avoid non-target effects, and the risk of horizontal gene transfer needs to be assessed. Not surprisingly many bacteria associated with insects (mainly those from the insect gut system) employ CPTPs naturally. Within the core microbial community of honey bees and bumble bees, genome studies revealed that several bacterial species have T6SS loci and effectors/toxins (e.g. Rhs proteins) for which expression was confirmed by transcriptome analysis; namely Apibacter spp. (Kwong et al., 2018), Candidatus Schmidhempelia bombi (Martinson et al., 2014), Frischella perrara (Engel et al., 2015), Gilliamella apicola and Snodgrassella alvi (Kwong et al., 2014). The huge variety of T6SS classes and diversification of related toxins among strains and species might mediate cell-cell interactions and help colonization of the different sections of the gut system. The antagonistic interaction of bacterial species via T6SSs may have finally resulted in (i) the coevolution of the bacteria within the host-associated microbial communities and (ii) an active defence system against invading microorganisms (Kwong et al., 2014; Steele et al., 2017; Steele et al., 2021). In particular, resistance to invasion by potential pathogens (e.g. Serratia sp. and other Enterobacteriaceae) (Steele et al., 2017) and the exclusion of potential commensals in metabolic interactions are enhanced by T6SS specificity. Recently, it was shown that the core community of the honey bee gut microbiome possessing T6SSs can rapidly eliminate the opportunistic pathogen Serratia marcescens, with limited negative effects on the bee symbionts (Steele et al., 2021). Noteworthy, S. marcescens has a T6SS itself, which helps the pathogen to persist in the bee gut (Steele et al., 2021). This observation inspires the idea of using symbiotic strains as probiotics against pathogenic bacteria. Further interaction with pathogenic organisms and their extracellular contractile injection systems (eCISs), e.g. Paenibacillus larvae causing American foulbrood (AFB) disease, cannot be excluded, but have not been studied yet.
Astonishing observations made on natural insecticidal effects of bacterial strains, turned out to be caused by bacterial T6SSs, Afps or PVCs. Other studies reported bactericidal and fungicidal effects of tailocins and other CPTPs (reviewed by Ghequire & de Mot, 2015; Patz et al., 2019). The fact, that CPTPs have been discovered in mutualistic, commensal and pathogenic bacteria is not surprising, since bacteria benefit from producing different types of CPTPs in competitive environments. Nowadays genome analyses tools are sufficiently robust to inform us on the symbiotic and pathogenic potential of newly discovered bacteria and enable us to select interesting CPTP-producing candidates from an ever-increasing range of fully sequenced strains. Thus, in our opinion, CPTPs may offer alternative, sustainable and safe solutions to a range of threats imposed by plant pathogens and herbivorous insect pests.
Based on the increasing availability of genetic/genomic information we are currently developing bioinformatical algorithms for predicting the presence/distribution of all known and yet unknown CPTPs in extensively studied and still insufficiently investigated bacteria, in a joined project of JKI-AG and the University of Tübingen. Thus, we are analysing in total ~72,000 sequenced bacteria from the JGI/IMG server regarding their CPTPs and are currently optimizing the algorithm’s accuracy. The CPTP-Pred algorithm annotates for bacterial strains, based on their protein-coding sequences of the entire genome, all possible CPTPs including their closest database hits. We are collecting all data on contractile phage tail-like particles, their hosts environments, and symbiotic or virulence potential, in a database termed CPTP-db. Also links to external databases, like eCIStem, that lists only eCISs, are provided (Geller et al., 2021). CPTP-db and CPTP-Pred will enable educated guesses and research hypotheses for targeted and highly specific applications of CPTP-producing bacteria against insect plant pests, bacterial and fungal phytopathogens and against pathogens of bees.
Using bacteria with fully sequenced genomes only, may appear a heavy constraint while searching for promising candidate bacteria, however, our approach based on in silico-analyses is meant to incorporate also all relevant genes (plant-beneficial and plant-pathogenic genes likewise) and not only those responsible for CPTP formation. The number of fully sequenced bacterial genomes is increasing rapidly and currently approximately 10,300 genomes of bacterial strains are listed as CPTP producers (excluding T6SS producers) in the CPTP-db. A more detailed view reveals approx. 6.200 R-type tailocin- and 4700 eCIS-producing strains. Only around a tenth of R-type tailocin producing strains seem to habour an additional eCIS. Interestingly, Geller and co-workers identified 1,071 eCIS-containing strains only, when using a more restricted list of toxins compared to our analysis (Geller et al., 2021). However, their novel toxins are included in our algorithm and we are able to identify all of their declared eCISs. All services are available via the new web resource PLaBAse (https://plabase.informatik.uni-tuebingen.de/pb/plabase.php) which provides among others (i) a database for screening approx. 5,600 plant-associated bacteria (PLaBA-db) and (ii) a plant growth-promoting traits prediction tool for bacterial genomes (PGPT-Pred). Further we host a tool for prediction of bacterial plant association by marker gene prediction (PIFAR-Pred) (Martínez-García et al., 2016).
Learning from nature to develop environment-friendly insecticides is appealing. Especially the high specificity of CPTPs leaving non-target organisms unaffected, in theory, renders them superior to conventional chemical pesticides, which often work on a broad spectrum of organisms, causing severe collateral damage. The impact of CPTPs on plants and ecosystems has to be determined for guaranteeing safe application of CPTP-producing bacteria in crop production systems and forestry. The genetic equipment of the bacterial strain needs to be assessed in detail and allows exclusion of candidates that harbour virulence factors. Nevertheless, in vivo experiments have to follow in silico analyses to guarantee the innocuousness of any kind of plant protecting agent. In this article, we report solely findings on bacterial strains that have been isolated from natural environments, i.e. from ecosystems in which they have established as part of a prokaryotic and eukaryotic community. Nonetheless, introducing living organisms into a new environment may have a significant impact on the ecosystem. Although CPTPs have been observed to possess a narrow target spectrum, this has to be confirmed by comprehensive experiments for any microbial strain, before its application in the field, not to mention the long lasting testing phase before reaching a status as a marketable plant protection product. Harmful effects on non-target organisms have not been reported for CPTPs yet, however, to advise caution should be good scientific practice in all approaches dealing with plant protecting agents. Accordingly, the two following goals should be pursued: (i) the development of environment-friendly plant protection products requires exact determination of targets and (ii) a human-induced enrichment of bacterial strains should be reversed by the native microbiota to minimize their environmental impact.
More and more evidence is produced that CPTP-producing bacteria dominate in a vast range of ecosystems and are highly abundant in our own microbiome. A recent study by Rojas and co-workers found that Bacteroidales bacteria from the human microbiome encode an eCIS, which the authors termed “Bacteroidales injection systems (BIS)”, that is present in the gut microbiomes of 99% of individuals from the United States and Europe. Remarkably, BIS genes are more prevalent in the gut microbiomes of healthy individuals than in those individuals suffering from inflammatory bowel disease (Rojas et al., 2020), suggesting that the native human microbiome employs CPTPs to defeat invading microorganisms. Research on application of tailocins as alternatives to conventional antibiotics for medicinal use is underway (Gebhart et al., 2015; Kirk et al., 2017).
The search for effective and environment-friendly insecticides requires new scientific approaches. Here, we have introduced the idea to employ contractile phage tail-like particles (CPTPs) in pest control, substantiated by convincing studies, ranging from fundamental research on CPTPs deciphering their atomic structures and their molecular mode of action to successful application. Numerous first-grade publications in high-ranking journals have provided plenty of evidence that CPTPs are promising alternatives to conventional chemical pesticides.
The author(s) declare that they do not have any conflicts of interest.
Balogh, B., J.B. Jones, F.B. Iriarte, M.T. Momol, 2010: Phage therapy for plant disease control. Current pharmaceutical biotechnology 11 (1), 48–57, DOI: 10.2174/138920110790725302.
Basler, M., 2015: Type VI secretion system: secretion by a contractile nanomachine. Philosophical Transactions of the Royal Society B: Biological Sciences 370 (1679), DOI: 10.1098/rstb.2015.0021.
Becker, M., S. Patz, Y. Becker, B. Berger, M. Drungowski, B. Bunk, J. Overmann, C. Spröer, J. Reetz, G.V. Tchuisseu Tchakounte, S. Ruppel, 2018: Comparative Genomics Reveal a Flagellar System, a Type VI Secretion System and Plant Growth-Promoting Gene Clusters Unique to the Endophytic Bacterium Kosakonia radicincitans. Frontiers in Microbiology 9, 1997, DOI: 10.3389/fmicb.2018.01997.
Böck, D., J.M. Medeiros, H.-F. Tsao, T. Penz, G.L. Weiss, K. Aistleitner, M. Horn, M. Pilhofer, 2017: In situ architecture, function, and evolution of a contractile injection system. Science 357 (6352), 713–717, DOI: 10.1126/science.aan7904.
Brackmann, M., S. Nazarov, J. Wang, M. Basler, 2017: Using Force to Punch Holes: Mechanics of Contractile Nanomachines. Trends in cell biology 27 (9), 623–632, DOI: 10.1016/j.tcb.2017.05.003.
Buttimer, C., O. McAuliffe, R.P. Ross, C. Hill, J. O’Mahony, A. Coffey, 2017: Bacteriophages and Bacterial Plant Diseases. Frontiers in Microbiology 8, 34, DOI: 10.3389/fmicb.2017.00034.
Carim, S., A.L. Azadeh, A.E. Kazakov, M.N. Price, P.J. Walian, L.M. Lui, T.N. Nielsen, R. Chakraborty, A.M. Deutschbauer, V.K. Mutalik, A.P. Arkin, 2021: Systematic discovery of pseudomonad genetic factors involved in sensitivity to tailocins. The ISME journal 15 (8), 2289–2305, DOI: 10.1038/s41396-021-00921-1.
Chang, Y.-W., L.A. Rettberg, D.R. Ortega, G.J. Jensen, 2017: In vivo structures of an intact type VI secretion system revealed by electron cryotomography. EMBO reports 18 (7), 1090–1099, DOI: 10.15252/embr.201744072.
Chen, L., Y. Zou, P. She, Y. Wu, 2015: Composition, function, and regulation of T6SS in Pseudomonas aeruginosa. Microbiological research 172, 19–25, DOI: 10.1016/j.micres.2015.01.004.
Deveau, A., G. Bonito, J. Uehling, M. Paoletti, M. Becker, S. Bindschedler, S. Hacquard, V. Hervé, J. Labbé, O.A. Lastovetsky, S. Mieszkin, L.J. Millet, B. Vajna, P. Junier, P. Bonfante, B.P. Krom, S. Olsson, J.D. van Elsas, L.Y. Wick, 2018: Bacterial-fungal interactions: ecology, mechanisms and challenges. FEMS Microbiology Reviews 42 (3), 335–352, DOI: 10.1093/femsre/fuy008.
Dorosky, R.J., J.M. Yu, L.S. Pierson, E.A. Pierson, 2017: Pseudomonas chlororaphis Produces Two Distinct R-Tailocins That Contribute to Bacterial Competition in Biofilms and on Roots. Applied and Environmental Microbiology 83 (15), DOI: 10.1128/AEM.00706-17.
Dreyer, J., A.P. Malan, L.M.T. Dicks, 2018: Bacteria of the Genus Xenorhabdus, a Novel Source of Bioactive Compounds. Frontiers in Microbiology 9, 3177, DOI: 10.3389/fmicb.2018.03177.
Engel, P., M.I. Vizcaino, J.M. Crawford, 2015: Gut symbionts from distinct hosts exhibit genotoxic activity via divergent colibactin biosynthesis pathways. Applied and Environmental Microbiology 81 (4), 1502–1512, DOI: 10.1128/AEM.03283-14.
Fernandez, M., A. Godino, A. Príncipe, G.M. Morales, S. Fischer, 2017: Effect of a Pseudomonas fluorescens tailocin against phytopathogenic Xanthomonas observed by atomic force microscopy. Journal of Biotechnology 256, 13–20, DOI: 10.1016/j.jbiotec.2017.07.002.
Ge, P., D. Scholl, P.G. Leiman, X. Yu, J.F. Miller, Z.H. Zhou, 2015: Atomic structures of a bactericidal contractile nanotube in its pre- and postcontraction states. Nature Structural & Molecular Biology 22 (5), 377–382, DOI: 10.1038/nsmb.2995.
Gebhart, D., S. Lok, S. Clare, M. Tomas, M. Stares, D. Scholl, C.J. Donskey, T.D. Lawley, G.R. Govoni, 2015: A modified R-type bacteriocin specifically targeting Clostridium difficile prevents colonization of mice without affecting gut microbiota diversity. mBio 6 (2), DOI: 10.1128/mBio.02368-14.
Geller, A.M., I. Pollin, D. Zlotkin, A. Danov, N. Nachmias, W.B. Andreopoulos, K. Shemesh, A. Levy, 2021: The extracellular contractile injection system is enriched in environmental microbes and associates with numerous toxins. Nature Communications 12 (1), 3743, DOI: 10.1038/s41467-021-23777-7.
Ghequire, M.G.K., R. de Mot, 2015: The Tailocin Tale: Peeling off Phage Tails. Trends in Microbiology 23 (10), 587–590, DOI: 10.1016/j.tim.2015.07.011.
Glick, B.R., 2012: Plant growth-promoting bacteria: mechanisms and applications. Scientifica 2012, 963401, DOI: 10.6064/2012/963401.
Haggård-Ljungquist, E., C. Halling, R. Calendar, 1992: DNA sequences of the tail fiber genes of bacteriophage P2: evidence for horizontal transfer of tail fiber genes among unrelated bacteriophages. Journal of Bacteriology 174 (5), 1462–1477, DOI: 10.1128/jb.174.5.1462-1477.1992.
Hinchliffe, S.J., 2013: Insecticidal Toxins from the Photorhabdus and Xenorhabdus Bacteria. The Open Toxinology Journal 3 (1), 101–118, DOI: 10.2174/1875414701003010101.
Ho, B.T., T.G. Dong, J.J. Mekalanos, 2014: A view to a kill: the bacterial type VI secretion system. Cell Host & Microbe 15 (1), 9–21, DOI: 10.1016/j.chom.2013.11.008.
Hurst, M.R.H., S.S. Beard, T.A. Jackson, S.M. Jones, 2007: Isolation and characterization of the Serratia entomophila antifeeding prophage. FEMS Microbiology Letters 270 (1), 42–48, DOI: 10.1111/j.1574-6968.2007.00645.x.
Hurst, M.R.H., A. Beattie, S.A. Jones, A. Laugraud, C. van Koten, L. Harper, 2018: Serratia proteamaculans Strain AGR96X Encodes an Antifeeding Prophage (Tailocin) with Activity against Grass Grub (Costelytra giveni) and Manuka Beetle (Pyronota Species) Larvae. Applied and Environmental Microbiology 84 (10), DOI: 10.1128/AEM.02739-17.
Jackson, T.A., D.G. Boucias, J.O. Thaler, 2001: Pathobiology of amber disease, caused by Serratia Spp., in the New Zealand grass grub, Costelytra zealandica. Journal of invertebrate pathology 78 (4), 232–243, DOI: 10.1006/jipa.2002.5078.
Jiang, F., N. Li, X. Wang, J. Cheng, Y. Huang, Y. Yang, J. Yang, B. Cai, Y.-P. Wang, Q. Jin, N. Gao, 2019: Cryo-EM Structure and Assembly of an Extracellular Contractile Injection System. Cell 177 (2), 370-383.e15, DOI: 10.1016/j.cell.2019.02.020.
Kirk, J.A., D. Gebhart, A.M. Buckley, S. Lok, D. Scholl, G.R. Douce, G.R. Govoni, R.P. Fagan, 2017: New class of precision antimicrobials redefines role of Clostridium difficile S-layer in virulence and viability. Science translational medicine 9 (406), DOI: 10.1126/scitranslmed.aah6813.
Kondo, Y.R., A.P. Primon, A.C.C.L. Da Fioreze, S.P. Da Cruz, 2020: Growth promotion of genetically improved Pinus taeda seedlings by inoculation with species of Bacillus. CERNE 26 (4), 456–462, DOI: 10.1590/01047760202026042757.
Kupferschmied, P., T. Chai, P. Flury, J. Blom, T.H.M. Smits, M. Maurhofer, C. Keel, 2016: Specific surface glycan decorations enable antimicrobial peptide resistance in plant-beneficial pseudomonads with insect-pathogenic properties. Environmental Microbiology 18 (11), 4265–4281, DOI: 10.1111/1462-2920.13571.
Kwong, W.K., P. Engel, H. Koch, N.A. Moran, 2014: Genomics and host specialization of honey bee and bumble bee gut symbionts. Proceedings of the National Academy of Sciences 111 (31), 11509–11514, DOI: 10.1073/pnas.1405838111.
Kwong, W.K., M.I. Steele, N.A. Moran, 2018: Genome Sequences of Apibacter spp., Gut Symbionts of Asian Honey Bees. Genome Biology and Evolution 10 (4), 1174–1179, DOI: 10.1093/gbe/evy076.
Lavermicocca, P., S.L. Lonigro, F. Valerio, A. Evidente, A. Visconti, 2002: Reduction of olive knot disease by a bacteriocin from Pseudomonas syringae pv. ciccaronei. Applied and Environmental Microbiology 68 (3), 1403–1407, DOI: 10.1128/AEM.68.3.1403-1407.2002.
Lavine, M.D., M.R. Strand, 2002: Insect hemocytes and their role in immunity. Insect Biochemistry and Molecular Biology 32 (10), 1295–1309, DOI: 10.1016/S0965-1748(02)00092-9.
Le, N.-H., V. Pinedo, J. Lopez, F. Cava, M.F. Feldman, 2021: Killing of Gram-negative and Gram-positive bacteria by a bifunctional cell wall-targeting T6SS effector. Proceedings of the National Academy of Sciences 118 (40), DOI: 10.1073/pnas.2106555118.
Lee, G., U. Chakraborty, D. Gebhart, G.R. Govoni, Z.H. Zhou, D. Scholl, 2016: F-Type Bacteriocins of Listeria monocytogenes: a New Class of Phage Tail-Like Structures Reveals Broad Parallel Coevolution between Tailed Bacteriophages and High-Molecular-Weight Bacteriocins. Journal of Bacteriology 198 (20), 2784–2793, DOI: 10.1128/JB.00489-16.
Lopes, M.J.d.S., M.B. Dias-Filho, E.S.C. Gurgel, 2021: Successful Plant Growth-Promoting Microbes: Inoculation Methods and Abiotic Factors. Frontiers in Sustainable Food Systems 5, 606454, DOI: 10.3389/fsufs.2021.606454.
Martínez-García, P.M., E. López-Solanilla, C. Ramos, P. Rodríguez-Palenzuela, 2016: Prediction of bacterial associations with plants using a supervised machine-learning approach. Environmental Microbiology 18 (12), 4847–4861, DOI: 10.1111/1462-2920.13389.
Martinson, V.G., T. Magoc, H. Koch, S.L. Salzberg, N.A. Moran, 2014: Genomic features of a bumble bee symbiont reflect its host environment. Applied and Environmental Microbiology 80 (13), 3793–3803, DOI: 10.1128/AEM.00322-14.
Monjarás Feria, J., M.A. Valvano, 2020: An Overview of Anti-Eukaryotic T6SS Effectors. Frontiers in Cellular and Infection Microbiology 10, 584751, DOI: 10.3389/fcimb.2020.584751.
Patz, S., Y. Becker, K.R. Richert-Pöggeler, B. Berger, R. Ruppel, D. H. Huson, M. Becker, 2019: Phage tail-like particles are versatile bacterial nanomachines – A mini-review. Journal of Advanced Research 19, 75-84, DOI: 10.1016/j.jare.2019.04.003.
Penz, T., S. Schmitz-Esser, S.E. Kelly, B.N. Cass, A. Müller, T. Woyke, S.A. Malfatti, M.S. Hunter, M. Horn, 2012: Comparative genomics suggests an independent origin of cytoplasmic incompatibility in Cardinium hertigii. PLoS genetics 8 (10), e1003012, DOI: 10.1371/journal.pgen.1003012.
Príncipe, A., M. Fernandez, M. Torasso, A. Godino, S. Fischer, 2018: Effectiveness of tailocins produced by Pseudomonas fluorescens SF4c in controlling the bacterial-spot disease in tomatoes caused by Xanthomonas vesicatoria. Microbiological research 212-213, 94–102, DOI: 10.1016/j.micres.2018.05.010.
Rojas, M.I., G.S. Cavalcanti, K. McNair, S. Benler, A.T. Alker, A.G. Cobián-Güemes, M. Giluso, K. Levi, F. Rohwer, B.A. Bailey, S. Beyhan, R.A. Edwards, N.J. Shikuma, 2020: A Distinct Contractile Injection System Gene Cluster Found in a Majority of Healthy Adult Human Microbiomes. mSystems 5 (4), DOI: 10.1128/mSystems.00648-20.
Scholl, D., 2017: Phage Tail-Like Bacteriocins. Annual review of virology 4 (1), 453–467, DOI: 10.1146/annurev-virology-101416-041632.
Shikuma, N.J., M. Pilhofer, G.L. Weiss, M.G. Hadfield, G.J. Jensen, D.K. Newman, 2014: Marine tubeworm metamorphosis induced by arrays of bacterial phage tail-like structures. Science 343 (6170), 529–533, DOI: 10.1126/science.1246794.
Smith, W.P.J., A. Vettiger, J. Winter, T. Ryser, L.E. Comstock, M. Basler, K.R. Foster, 2020: The evolution of the type VI secretion system as a disintegration weapon. PLOS Biology 18 (5), e3000720, DOI: 10.1371/journal.pbio.3000720.
Speare, L., M. Woo, A. K. Dunn, A. N. Septer, 2021: A large lipoprotein mediates target specificity for T6SS-dependent killing, DOI: 10.1101/2021.04.26.440508.
Steele, M.I., W.K. Kwong, M. Whiteley, N.A. Moran, 2017: Diversification of Type VI Secretion System Toxins Reveals Ancient Antagonism among Bee Gut Microbes. mBio 8 (6), DOI: 10.1128/mBio.01630-17.
Steele, M.I., E. V.S. Motta, T. Gattu, D. Martinez, N.A. Moran, 2021: The Gut Microbiota Protects Bees from Invasion by a Bacterial Pathogen. Microbiology spectrum 9 (2), e0039421, DOI: 10.1128/Spectrum.00394-21.
Taylor, N.M.I., N.S. Prokhorov, R.C. Guerrero-Ferreira, M.M. Shneider, C. Browning, K.N. Goldie, H. Stahlberg, P.G. Leiman, 2016: Structure of the T4 baseplate and its function in triggering sheath contraction. Nature 533 (7603), 346–352, DOI: 10.1038/nature17971.
Trunk, K., J. Peltier, Y.-C. Liu, B.D. Dill, L. Walker, N.A.R. Gow, M.J.R. Stark, J. Quinn, H. Strahl, M. Trost, S.J. Coulthurst, 2018: The type VI secretion system deploys antifungal effectors against microbial competitors. Nature Microbiology 3 (8), 920–931, DOI: 10.1038/s41564-018-0191-x.
Vacheron, J., C.M. Heiman, C. Keel, 2021: Live cell dynamics of production, explosive release and killing activity of phage tail-like weapons for Pseudomonas kin exclusion. Communications biology 4 (1), 87, DOI: 10.1038/s42003-020-01581-1.
Vacheron, J., M. Péchy-Tarr, S. Brochet, C.M. Heiman, M. Stojiljkovic, M. Maurhofer, C. Keel, 2019: T6SS contributes to gut microbiome invasion and killing of an herbivorous pest insect by plant-beneficial Pseudomonas protegens. The ISME journal 13 (5), 1318–1329, DOI: 10.1038/s41396-019-0353-8.
Vlisidou, I., A. Hapeshi, J.R. Healey, K. Smart, G. Yang, N.R. Waterfield, 2019: The Photorhabdus asymbiotica virulence cassettes deliver protein effectors directly into target eukaryotic cells. eLife 8, DOI: 10.7554/eLife.46259.
Yang, G., A.J. Dowling, U. Gerike, R.H. ffrench-Constant, N.R. Waterfield, 2006: Photorhabdus virulence cassettes confer injectable insecticidal activity against the wax moth. Journal of Bacteriology 188 (6), 2254–2261, DOI: 10.1128/JB.188.6.2254-2261.2006.
Yao, G.W., I. Duarte, T.T. Le, L. Carmody, J.J. LiPuma, R. Young, C.F. Gonzalez, 2017: A Broad-Host-Range Tailocin from Burkholderia cenocepacia. Applied and Environmental Microbiology 83 (10), DOI: 10.1128/AEM.03414-16.