

Journal für Kulturpflanzen, 74 (11-12). S. 257–262, 2022 | DOI: 10.5073/JfK.2022.11-12.05 | Töpfer and Trapp
200 years Mendel: the grapevine breeding perspective
200 Jahre Mendel: die Perspektive der Rebenzüchtung
 | (c) The author(s) 2022 This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/deed.en). |
Submitted/accepted for publication: 28 September 2022/14 November 2022 |
Mendelian rules in grapevine breeding remained hidden for a long time in the complexity of traits and difficulties of handling the crop. A few traits with minor relevance for breeders had been genetically dissected like berry color or autumn leaf color. Mildew resistance and wine quality have been and are still the most relevant traits. Resistant cultivars developed during the first half of the 20th century failed to convince the quality demand of the market. Since the turn of the millennium, a new generation of fungus-resistant new breeds in Germany gives hope to viticulture in times of climate change and a rising debate on sustainability. It took the breeding effort and continuity of generations of breeders, finally far more than 120 years, to provide the market with cultivars convincing with wine quality, the most complex and debated trait in viticulture. Due to a lack of genetic knowledge, selection in grapevine breeding was done mostly without Mendelian genetics schemes. However, the logistic restrictions (field and greenhouse space, labor, and time) in grapevine breeding require efficient selection schemes based on Mendelian traits. In the recent past, marker-assisted selection (MAS) opened a new chapter in grapevine breeding. For many powdery and downy mildew resistance loci MAS is possible today. Using these tools, stacking of resistance loci according to Mendelian rules can be achieved. The concept of developing and using – for resistances – locus-specific homozygous lines (LSH-lines) permits a new attempt to create large progenies and to select the offspring for all traits other than resistance. New markers and selection concepts for more complex traits (e.g. moderate yield, phenology, Botrytis resilience, quality parameters) are urgently needed in practical breeding. Only such tools allow the acceleration of selection at the stage of young seedlings (MAS) and the resulting plants. To that end, newly emerging phenotyping tools are required.
Vitis, grapevine breeding, quality, resistance, phenotyping
Die Mendelschen Regeln blieben in der Rebenzüchtung lange in der Komplexität der Merkmale und Schwierigkeiten im Umgang mit dieser Kulturpflanze verborgen. Für einige wenige Merkmale, die für Züchter größtenteils nicht wirklich relevant sind, wurde die Genetik geklärt, wie die Beerenfarbe oder die Herbstfarbe der Blätter. Für die Züchtung waren und sind Mehltauresistenz und Weinqualität die wichtigsten Merkmale. Resistente Sorten, die in der ersten Hälfte des 20. Jahrhunderts entwickelt wurden, konnten den Qualitätsansprüchen des Marktes nicht genügen. Seit der Jahrtausendwende existiert eine neue Generation pilzwiderstandsfähiger Neuzüchtungen in Deutschland, die dem Weinbau in Zeiten des Klimawandels und einer aufkommenden Nachhaltigkeitsdebatte Hoffnung gibt. Es bedurfte der züchterischen Anstrengung und Kontinuität von Generationen von Züchtern, schließlich weit mehr als 120 Jahre, um den Markt mit Sorten zu versorgen, die hinsichtlich ihrer Weinqualität, dem komplexesten und umstrittensten Merkmal im Weinbau, überzeugen. Aufgrund fehlender genetischer Kenntnisse erfolgte die Selektion meist ohne die systematische Anwendung der Mendelschen Regeln. Die logistischen Beschränkungen (Feld- und Gewächshausfläche, Arbeit und Zeit) in der Rebenzüchtung erfordern jedoch effiziente Selektionsschemata auf der Grundlage der Mendelschen Erkenntnisse. In der jüngeren Vergangenheit schlägt die markergestützte Selektion (MAS) ein neues Kapitel für die Rebenzüchtung auf. Für viele Resistenzloci gegen Echten und Falschen Mehltau ist heute MAS möglich. Mit diesen Werkzeugen ist eine Kombination von Resistenzgenorten gemäß den Mendelschen Regeln möglich. Das Konzept, Locus-spezifische homozygote Linien (LSH-Linien) für Resistenzen zu entwickeln und zu verwenden, erlaubt einen neuen Versuch, große Nachkommenschaften zu schaffen und diese Nachkommen auf alle anderen relevante Merkmale zu selektieren. Daher werden neue Marker und Selektionskonzepte für komplexere Merkmale (z. B. moderater Ertrag, Phänologie, Botrytis-Widerstandsfähigkeit, Qualitätsparameter) dringend benötigt. Nur solche Werkzeuge erlauben die akut notwendige Beschleunigung der Selektion im Stadium junger Sämlinge (MAS) und der daraus resultierenden Pflanzen. Zu diesem Zweck sind neu zu entwickelnde Phänotypisierungsverfahren erforderlich.
Vitis, Rebenzüchtung, Qualität, Resistenz, Phänotypisierung
The accidental introduction of powdery mildew (PM, Erysiphe necator, 1845), phylloxera (Daktulosphaira vitifoliae, 1863) and downy mildew (DM, Plasmopara viticola, 1878) to Europe in the second half of the 19th century posed the existence of multi-thousand-year-old viticulture into question. Plant protection with sulfur and copper ensured the survival of viticulture controlling the mildews. But more happened: the pathogens triggered intense private breeding activities in particular in France but also Hungary. It finally resulted in a solution for viticulture by developing rootstocks (American Vitis species or interspecific hybrids thereof) tolerant against phylloxera and adapted to the European soils (Manty, 2006). However, quality deficits of scion varieties caused a deep disappointment and led to prejudices still persisting today.
The important French breeders Albert Seibel (1844-1936), Georges Couderc (1850-1928), Eugene Kuhlmann (1858-1932), Bertille Seyve (father, 1864-1939), Francois Baco (1865-1947), Bertille Seyve (son, 1895-1959), Joannes Seyve (1900-1966), and many others (Reynolds, 2015) developed numerous varieties and breeding lines largely by mass selection. When most of these breeders had active breeding programmes, Gregor Mendel´s (1822-1884) publication “Versuche über Pflanzen-Hybriden“ (Experiments on plant hybrids) (Mendel, 1866) was unknown. This cornerstone on genetics was lost for decades until Hugo de Vries (1848-1935), Carl Correns (1864-1933), and Erich Tschermak (1871-1962) independently rediscovered and published their experimental results. This new information remained unknown for most grapevine breeders. However, their observations were assimilated quickly by geneticists such as Erwin Baur (1875-1933). Thus, he proposed in 1914 to make use of F2 plants instead of a grapevine F1 offspring to select new cultivars (Baur, 1922). From crosses of Vitis vinifera cultivars with downy mildew and phylloxera-resistant American Vitis species, he suggested to start selection at the F2 generation. At that time 5-7 million F2 plants annually were placed under downy mildew selection in the greenhouse at Müncheberg (close to Berlin) (Baur, 1933). A further selection was done in a cold frame to verify downy mildew resistance. Some 25,000 seedlings remained. Baur finally claimed that one out of 5,000 plants would be acceptable for its wine quality (Baur, 1933). His description is indicative for the low selection efficiency and slow progress in grapevine breeding in order to identify genotypes, that give sufficient yield, show resistance against powdery and downy mildew as well as suitable phenological adaptation, and most important a good wine quality. Accepting the success of rootstock breeding in Hungary, Austria, and France, Erwin Baur split the breeding goals – instead of direct producers being phylloxera and mildew-resistant – to select for mildew-resistant scions and phylloxera-resistant rootstocks (Baur, 1933). Bernhard Husfeld (1900-1970), Baur´s coworker, stated a few years later that F2 plants or backcrosses are most promising for successful selection of wine grape cultivars (Husfeld, 1939).
The first trials to make use of the Mendelian rules go back to researchers and breeders that often made interspecific crosses. It is well known that Vitis species are interfertile and the intention to combine quality and resistances resulted in both intraspecific crosses within the species Vitis vinifera but also many crosses between Vitis species so called ICs (interspecific crosses). One of the first reports on a genetic dissection of traits is the report of Hedrick & Anthony (1915). The authors described the method of crossing as follows: „The female blossoms are emasculated before the calyx cap splits off and are then bagged; the male blossoms are also bagged before the calyx splits. When the pollen is ripe, the bagged male cluster is usually cut from the vine and all or part of it brushed over the emasculated female.“ Today the protocol is quite the same but the knowledge about the genetics has improved. Hedrick and Anthony reported already in 1915 about their results in the light of Mendel`s laws and surely were one of the first who gave clear cut evidence of Mendelian inheritance of certain traits. On their retrospective view on 25 years of breeding they pointed towards several problems in dissecting the genetics of grapevine arising from
low number of seedlings,
seedlings with low vigor (inbreeding depression),
complexity of numerous traits in grapevine breeding,
self-fertilization that might have had occurred in some cases though emasculation was performed.
A major finding concerns inbreeding depression, which was indicated by Hedrick & Anthony (1915) resuming on the analysis of nearly 3000 selfed varieties by stating: “These seedlings have thrown much light upon the inheritance of various factors, but they have been so uniformly lacking in vigor as to lead to the belief that only through crossbreeding can we hope to produce improved varieties”. Hedrick & Anthony (1915) studied various traits such as self-sterility (unclear results), flower sex (unclear results), berry size (no clear tendency), berry form (eventually intermediate), season of ripening (impact of many factors).
Years later, in his review on genetics and grapevine breeding, Husfeld (1939) summarized the knowledge of Mendelian traits. Only a very few traits had been elucidated. Often these traits are of minor relevance for application in viticulture: the inheritance of some color traits (berry color or autumn leaf color) and leaf traits (petiole sinus or hairs on the shoot tip) as monogenic traits or shoot tip traits as digenetic traits in the F2 of interspecific crosses. Nevertheless, at that early stage of genetic dissection of traits, in some cases the genetic architecture became clear.
The genetic analyses of berry color gave clear-cut evidence of a dominant (black) and recessive (white) inheritance of color formation (Hedrick & Anthony 1915). The trait is even more complex, as varieties exist with various colors distinguished by using OIV descriptor 225 as green yellow (white) – rose – red – grey – dark red violet – blue black. Today we know that different transposon-induced mutants of the myb gene (e.g. Kobayashi et al., 2004, Walker et al., 2007, Röckel et al., 2020, Röckel et al., 2022), but also different enzymes of the anthocyanin pathway, cause the various berry color types in complex ways.
Inheritance of flower sex in grapevine is somewhat complex. Vitis wild species are dioecious showing either female or male flowers. In contrast, most traditional as well as newly selected cultivars (Vitis vinifera ssp. vinifera) produce hermaphroditic flowers, which are the fundamental characteristic for yield stability and surely an early and an important trait during domestication. Already prior to Mendel’s work, different flower types had been identified within the genus Vitis (Walter, 1788; Michaux, 1803; De Candolle, 1824 as cited by Negrul, 1936). Negrul (1936) summarized the knowledge of the genetic structure of the flower sex locus: Hedrick & Anthony (1915) observed a 1:1 (male: hermaphrodite) segregation. Valleau (1916) first proposed three alleles. Today female (ff), hermaphrodite (HH, Hf) and male (MH, Mf) haplotypes at one locus and dominance of MH or Mf > HH or Hf > ff are distinguished. The flower sex trait is of particular interest for breeders as Hf or HH haplotypes show a higher yield stability compared to female (ff) genotypes. Technically, crosses with female genotypes (ff) as mother plants are easy to perform, as there is no need for laborious emasculations.
Analysing the inheritance of flower sex locus, which is located on chromosome two, Fechter et al. (2012) identified a closely linked SSR-marker. By using this or similar markers, the majority of cultivars and genotypes can be differentiated for their haplotypes at the locus. This information is very useful as the flower sex can be genetically analysed 3 years prior to first phenotypic expression of the trait. For some applications in practical breeding such as the development and use of locus-specific homozygous lines (LSH-Lines), the selection for flower sex could be very important. Recently, Töpfer & Trapp (2022) proposed to create LSH-lines that carry multiple resistance loci against one pathogen in a homozygous manner but remain heterozygous for most of the genome to avoid inbreeding depression. If these lines were female (ff), emasculation in a later step becomes obsolete and production of seeds will be simplified enormously. Crosses with homozygous hermaphroditic (HH) breeding lines (e.g. other LSH-Lines) or cultivars (e.g. Riesling) will result in a uniform (Hf) hermaphroditic F1 offspring. In other crossing schemes, the removal of female flowering plants would be possible as they are usually lower in yield, thus avoiding labor and costs of cultivation of undesired (ff) seedlings in the vineyard. Analysis of the flower sex locus in Vitis is progressing. Recently a candidate gene, VviPLATZ1 transcription factor, was identified as a key factor for female flower morphology formation (Iocco-Corena et al., 2021).
Mendelian inheritance of disease resistance was difficult to understand for a long time. In the beginning, grapevine breeders combined breeding lines of diverse genetic origin, i.e. they created and made use of interspecific hybrids of North American Vitis species and crossed them among each other or with V. vinifera cultivars. In such complex pedigrees, the origin of a resistance remained unknown for quite some time. Crosses were made and resulting seedlings were phenotyped for powdery mildew (PM) and downy mildew (DM) resistance. Exceptional was the work of Alain Bouquet giving the first example of a consequent introgression of a resistance locus from Muscadinia rotundifolia into V. vinifera (Pauquet et al., 2001). The authors developed introgression lines up to pBC4 (pseudo-backcross 4) by crossing lines selected for PM and DM resistance as well as other desired characters with different V. vinifera cultivars to stepwise replace parts of the wild species genome and simultaneously avoiding inbreeding depression (compare Fig., pedigree of VRH3082-1-42).
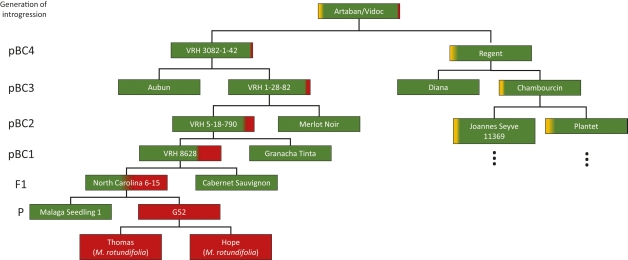
Fig. Pedigree of the new cultivars Artaban and Vidoc, which carry stacked resistance loci from Muscadinia rotundifolia (Pauquet et al., 2001) and American Vitis species coming from Chambourcin. The breeding line VRH3082-1-42 is derived from a systematic introgression into Vitis vinifera over 4 generations. The introgressed resistance locus confers a resistance against both powdery mildew (Run1) and downy mildew (Rpv1) and is inherited as single Mendelian trait. The development of the pBC4 (pseudo backcross) took about three to four decades. The pedigree of Chambourcin which contributes Ren3/Ren9 and Rpv3.1 (Di Gaspero et al., 2012) is going back to the 19th century and was recently updated by Röckel et al. (2021). Colors symbolize the following genomes: green = V. vinifera; red = M. rotundifolia, and yellow = American Vitis species. Red and yellow indicate introgressed parts of genomes.
A major breakthrough towards application of Mendelian genetics was achieved in grapevine breeding when genetic maps based on SSR markers (e.g. Merdinoglu et al., 2002) and the sequence of the reference genome of PN40024 (Jaillon et al., 2007) became available. Although not PM and DM-resistant, PN40024 proved to be an excellent tool for fine-mapping studies as it permits deriving of SSR marker sequences within genomic regions of interest and a subsequent testing in a different genetic background. These advances in grapevine genetics allowed detailed characterization of the resistances and their candidate genes. By mapping resistances, a paradigm shift was initiated (Thomas et al., 1993; Dalbo et al., 2001, Doligez et al., 2002), followed by the application of marker-assisted selection (MAS) in grapevine breeding (Eibach et al., 2007). Some resistance genes have been identified by gene transfer, converting regenerated transgenic plants from susceptible to resistant. The first resistance genes were described in 2013 as TIR-NB-LRR genes underlying the resistance of the Run1 and Rpv1 loci (Feechan et al., 2013) after detailed investigation of the genome and the individual resistance loci.
Today, numerous dominantly inherited loci for resistance against powdery mildew (e.g. Run1, Run2 etc. = resistance Uncinula necator and Ren1, Ren2, Ren3 etc. = resistance Erysiphe necator, respectively) and against downy mildew (e.g. Rpv1, Rpv3.1 etc. = resistance Plasmopara viticola) (see: Hausmann und Töpfer → “data on breeding and genetics” at www.vivc.de/loci) are known. Few of them are characterized in more detail and markers have been identified to be routinely used in MAS: e.g. for Run1, Ren1, Ren3, Ren4, Ren9 and Rpv1, Rpv3.1, Rpv3.2, Rpv3.3, Rpv10, Rpv12. These markers permit monitoring of the inheritance of loci as single Mendelian traits. The markers even permit the combination (stacking) of loci, thus building complex resistances consisting of e.g. 3 Ren and 3 Rpv loci (3 + 3) in order to increase durability of resistance. Currently the first cultivars carrying 2 + 2 are available for viticulturists e.g. Cabernet Cortis (Ren3, Ren9, Rpv3.3, Rvp10), Calardis Blanc (Ren3, Ren9, Rpv3.1, Rvp3.2), Sauvignac (Ren3, Ren9, Rpv3.1, Rvp12) (see www.vivc.de). In addition Artaban and Vidoc show 3 + 2 (Run1, Ren3, Ren9, Rvp1, Rpv3.1) resistance loci in combination (Fig.).
Beyond mildew resistances, other traits have been analysed such as resistance against black rot (Guignardia bidwellii) or Pierce Disease (Xylella fastidiosa). However, in many cases markers closely linked with the respective locus and useful for MAS remain to be developed. Generally speaking, markers have made many resistance loci accessible as Mendelian traits but the genetics of many other traits, e.g. yield, Botrytis resilience, and the all dominating trait of wine quality, remain to be elucidated. For further elucidation of the genetics of these important grapevine traits, the development of high-throughput phenotyping methods is needed. Two principal approaches can be distinguished: (1) phenotyping in lab or greenhouse and (2) field phenotyping. Several examples have been reported for each case and a few of them are advanced to be used routinely (e.g. Herzog et al., 2015; Mack et al., 2020; Zendler et al., 2021). Using such phenotypic data combined with genetic approaches like GWAS (genome wide association studies), it should be possible to find QTLs (quantitative trait loci) and markers for MAS for many traits (Flutre et al., 2022).
The simple application of Mendelian rules was not successful due to complexity of the traits that need to be combined in grapevine breeding. Geneticist Erwin Baur´s conclusion to have faster breeding success separating phylloxera-resistance from mildew resistance by proceeding with the concept of breeding for rootstocks and scions separately remained the successful way. Baur also suggested to focus on both mildews instead of other diseases. Husfeld proved that the combination of resistance and quality traits is possible. His cultivars ‘Aris’ and ‘Siegfriedrebe’ were milestones in grapevine breeding history but could not convince the winegrowers due to low yield and virus susceptibility. Husfeld’s successor, Gerhardt Alleweldt (1927-2005) and his team, selected the first cultivar (cv. ‘Regent’) from resistance breeding that is grown on a large area in Germany (up to 2,200 ha). Due to increased molecular knowledge in the last few decades, tremendous breeding progress has been achieved. Some of the complex traits in grapevine, in particular resistance traits, have been genetically dissected to such an extent that they can be treated as Mendelian traits by application of MAS. Modern molecular tools and techniques permit further analysis of other complex traits and help to reduce their complexity and with their consequent application, new breeding goals can be addressed. Facing a rapid climate change and the EU GREEN DEAL debate, viticulture will urgently need better performing cultivars.
The authors declare that they do not have any conflicts of interest.
Baur, E., 1922: Einige Aufgaben der Rebenzüchtung im Lichte der Vererbungswissenschaft. Beitr. Pflanzenzucht 5, 104-11, Vortrag, gehalten 1914.
Baur, E., 1933: Der heutige Stand der Rebenzüchtung in Deutschland. Der Züchter. Zeitschrift für theoretische und angewandte Genetik: 5 73-77, DOI: 10.1007/BF01812478.
Dalbo, M.A., G.N. Ye, N.F. Weeden, W.F. Wilcox, B.I. Reisch, 2001: Marker-assisted selection for powdery mildew resistance in grapes. Journal of the American Society of Horticultural Science 126 (1), 83-89, DOI: 10.21273/JASHS.126.1.83.
Di Gaspero, G., D. Copetti, C. Coleman, S.D. Castellarin, R. Eibach, P. Kozma, T. Lacombe, G. Gambetta, A. Zvyagin, P. Cindric, L. Kovacs, M. Morgante, R. Testolin, 2012: Selective sweep at the Rpv3 locus during grapevine breeding for downy mildew resistance. Theorectical and Applied Genetics 124, 277–286, DOI: 10.1007/s00122-011-1703-8.
Doligez, A., A. Bouquet, Y. Danglot, F. Lahogue, S. Riaz, C.P. Meredith, K.J. Edwards, P. This, 2002: Genetic mapping of grapevine (Vitis vinifera L.) applied to the detection of QTLs for seedlessness and berry weight. Theoretical and Applied Genetics 105, 780-795, DOI: 10.1007/s00122-002-0951-z.
Eibach, R., E. Zyprian, L. Welter, R. Töpfer, 2007: The use of molecular markers for pyramiding resistance genes in grapevine breeding. Vitis 46, 120-124, DOI: 10.5073/vitis.2007.46.120-124.
Fechter, I., L. Hausmann, M. Daum, T. Rosleff Sörensen, P. Viehöver, B. Weisshaar, R. Töpfer, 2012: Candidate genes within a 143 kb region of the flower sex locus in Vitis. Molecular Genetics and Genomics 287, 247-259, DOI: 10.1007/s00438-012-0674-z.
Feechan, A., C. Anderson, L. Torregrosa, A. Jermakow, P. Mestre, S. Wiedemann-Merdinoglu, D. Merdinoglu, A.R. Walker, L. Cadle-Davidson, B. Reisch, S. Aubourg, N. Bentahar, B. Shrestha, A. Bouquet, A.F. Adam-Blondon, M.R. Thomas, I.B. Dry, 2013: Genetic dissection of a TIR-NB-LRR locus from the wild North American grapevine species Muscadinia rotundifolia identifies paralogous genes conferring resistance to major fungal and oomycete pathogens in cultivated grapevine. The Plant Journal 76, 661-674, DOI: 10.1111/tpj.12327.
Flutre, T., L. Le Cunff, A. Fodor, A. Launay, C. Romieu, G. Berger, Y. Bertrand, N. Terrier, I. Beccavin, V. Bouckenooghe, M. Roques, L. Pinasseau, A. Verbaere, N. Sommerer, V. Cheynier, R. Bacilieri, J.-M. Boursiquot, T. Lacombe, V. Laucou, P. This, J.-P. Péros, A. Doligez, 2022: A genome-wide association and prediction study in grapevine deciphers the genetic architecture of multiple traits and identifies genes under many new QTLs. G3 Genes|Genomes|Genetics 12 (7), DOI: 10.1093/g3journal/jkac103.
Hedrick, U.P., R.D. Anthony, 1915: Inheritance of certain characters of grapes. New York Agricultural Experiment Station Bulletin, Geneva NY 45, 3-19.
Herzog, K., R. Wind, R. Töpfer, 2015: Impedance of the grape berry cuticle as a novel phenotypic trait to estimate resistance to Botrytis cinerea. Sensors 15, 12498-12512, DOI: 10.3390/s150612498.
Husfeld, B., 1939: Genetik und Rebenzüchtung. Agronomia Lusitana 1, 200-235.
Iocco-Corena, P., J. Chaïb, L. Torregrosa, D. Mackenzie, M.R. Thomas, H.M. Smith, 2021: VviPLATZ1 is a major factor that controls female flower morphology determination in grapevine. Nature Communications 12 (Art. 6995), DOI: 10.1038/s41467-021-27259-8.
Jaillon, O., J.M. Aury, B. Noel, A. Policriti, C. Clepet, A. Casagrande, N. Choisne, S. Aubourg, N. Vitulo, C. Jubin, A. Vezzi, F. Legeai, P. Hugueney, C. Dasilva, D. Horner, E. Mica, D. Jublot, J. Poulain, C. Bruyere, A. Billault, et al., 2007: The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449 (7161), 463-468, DOI: 10.1038/nature06148.
Kobayashi, S., N. Goto-Yamamoto, H. Hirochika, 2004: Retrotransposon-Induced Mutations in Grape Skin Color. Science 304, 982, DOI: 10.1126/science.109501.
Mack, J., F. Rist, K. Herzog, R. Töpfer, V. Steinhage, 2020: Constraint-based automated reconstruction of grape bunches from 3D range data for high-throughput phenotyping. Biosystems Engineering 197, 285-305, DOI: 10.1016/j.biosystemseng.2020.07.004.
Manty, F., 2006: Hintergründe zur Entstehung der Bezeichnung der Unterlagenselektionen von Sigmund Teleki und Franz Kober. Deutsches Weinbau-Jahrbuch 57, 159-164.
Mendel, G., 1866: Versuche über Pflanzen-Hybriden. Verhandlungen des Naturforschenden Vereins zu Brünn 4, 3-47.
Merdinoglu, D., S. Wiedemann-Merdinoglu, P. Coste, V. Dumas, S. Haetty, G. Butterlin, C. Greif, 2002: Genetic analysis of downy mildew resistance derived from Muscadinia rotundifolia. In: Hajdu, E., E. Borbas (eds) Proceedings of the 8th International Conference on Grape Genetics and Breeding. Kecskemet, Hungary, 26-31 August 2002, 603/I edn. International Society for Horticultural Science (ISHS), Leuven; Belgium, pp 451-456.
Negrul, A.M., 1936: Variabilität und Vererbung des Geschlechts bei der Rebe. Gartenbauwissenschaft 10, 215-231.
Pauquet, J. A. Bouquet, P. This, A.F. Adam-Blondon, 2001: Establishment of a local map of AFLP markers around the powdery mildew resistance gene Run1 in grapevine and assessment of their usefulness for marker assisted selection. Theoretical and Applied Genetics 103, 1201-1210, DOI: 10.1007/s001220100664.
Reynolds, A., 2015: Grapevine breeding in France a historical perspective. In: Reynolds, A.G. (ed) Grapevine Breeding Programs for the Wine Industry. Woodhead Publishing, 65-76.
Röckel, F., C. Moock, U. Braun, F. Schwander, P. Cousins, E. Maul, R. Töpfer, L. Hausmann, 2020: Color intensity of the red-fleshed berry phenotype of Vitis vinifera Teinturier grapes varies due to a 408 bp duplication in the promoter of VvmybA1. Genes 11 (8), 891, DOI: 10.3390/genes11080891.
Röckel, F., O. Trapp, E. Zyprian, L. Hausmann, D. Migliaro, S. Vezzulli, R. Töpfer, E. Maul, 2021: A 'Regent' pedigree update: ancestors, offspring and their confirmed resistance loci. Vitis 60, 189-193, DOI: 10.5073/vitis.2021.60.189-193.
Röckel, F., C. Moock, F. Schwander, E. Maul, R. Töpfer, L. Hausmann, 2022: A 69 kbp Deletion at the Berry Color Locus Is Responsible for Berry Color Recovery in Vitis vinifera L. Cultivar ‘Riesling Rot’. International Journal of Molecular Sciences 23: 3708, DOI: 10.3390/ijms23073708.
Thomas, M.R., P. Cain, S. Matsumoto, N. Steele Scott, 1993: Microsatellite sequences in grapevine for mapping and fingerprinting. Proceedings of the Australian-Japan Workshop on 'Techniques of Gene Diagnosis and Breeding in Fruit Trees', November 24-27, 1992, Tsukuba; Japan 7-13.
Töpfer, R., O. Trapp, 2022: A cool climate perspective on grapevine breeding: climate change and sustainability are driving forces for changing varieties in a traditional market. Theoretical and Applied Genetics, DOI: 10.1007/s00122-022-04077-0.
Valleau, W.D., 1916: Inheritance of sex in the grape. The American Naturalist 50, 554-564.
Walker, A.R., E. Lee, L. Bogs, D.A.J. McDavid, M.R. Thomas, S. Robinson, 2007: White grapes arose through the mutation of two similar and adjacent regulatory genes. Plant Journal 49, 772–785, DOI: 10.1111/j.1365-313X.2006.02997.x.
Zendler, D., N. Malagol, A. Schwandner, R. Töpfer, L. Hausmann, E. Zyprian, 2021: High-throughput phenotyping of leaf discs infected with grapevine downy mildew using shallow convolutional neural networks. Agronomy 11 (9), 1768, DOI: 10.3390/agronomy11091768.