VITIS: Vol. 63, Art. 4, 13 pp. (2024) | DOI: 10.5073/vitis.2024.63.04 | Alinejad et al.
Potassium silicate counteracts salt-induced damage associated with changes in some growth characteristics, physiological, biochemical responses, and nutrient contents in two grapevines (Vitis vinifera L.) cultivars
 | (c) The author(s) 2024 This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/deed.en). |
Submitted/accepted for publication: August, 18, 2022/January, 29, 2024 |
This study investigates the effects of potassium silicate on the growth characteristics, physiological parameters, biochemical parameters, and nutrient content of two grapevine cultivars, 'Bidaneh Ghermez' and 'Sahibi Gird', under NaCl stress conditions. The plants were exposed to NaCl solutions with concentrations of 0, 40, and 80 mM in a hydroponic system. Additionally, the plants were treated with potassium silicate sprays at concentrations of 0, 50, 100, and 200 mg L-1. As NaCl levels increased, both 'Bidaneh Ghermez' and 'Sahibi Gird' cultivars exhibited reduced fresh and dry root weights. However, with potassium silicate application at 200 mg L-1, the rate of root dry weight loss was reduced to 28% and 66.4% for 40 mM and 80 mM NaCl treatments, respectively. The maximum total protein content (1.65 mg L-1 fresh weight) was detected at the 80 mM NaCl level and potassium silicate application at 50 mg L-1. The maximum ascorbate peroxidase activity was observed at a potassium silicate concentration of 50 mg L-1. Based on the results, increasing NaCl levels significantly boosted plant Na+ percentage. In treatments with 40 and 80 mM NaCl (without potassium silicate), nitrate levels decreased by 32.34% and 46.71%, respectively, compared to the control. The amount of leaf iron in the 40 mM salinity treatment increased and by 10.47% with potassium silicate at a concentration of 200 mg L-1. The findings confirmed the role of potassium silicate in modulating the negative effects of NaCl, although more investigations in different grapevine cultivars under NaCl stress are required in this field.
Growth parameters, Osmolyte, Potassium, Sodium, Vitis vinifera L.
Salt stress is one of the main stressors that can lead to crop yield reductions (Zhu et al., 2019b). Grapevine (Vitis vinifera L.) is a valuable crop with global significance, ranking among the world's most important fruit crops. However, it faces significant viticultural challenges, particularly soil salinization, which affects nearly 6% of the Earth's land area (Cuena-Lombraña et al., 2022). Grapevines are typically grown in semi-arid regions, where drought and salinity are the most frequent issues (Cramer et al., 2007). Field studies suggest that grapevines should be classified as moderately salt-sensitive (Stevens et al. 1999). While salt tolerance primarily derives from plant genetics, it is also influenced by various factors, including the plant's growth stage and the presence of other stressors (Grigore and Vicente, 2023). Negrao et al. (2017) discovered that NaCl ions are collected in young leaves and older leaves of salt-sensitive and salt-tolerant cultivars, correspondingly. The build-up of NaCl ions in the roots causes nutritional distinctions in the root tissue due to a reduced K+-to-Na+ ratio, resulting in increased salt levels. Quickly detecting excessive Na+ signal is a precondition for beginning to restore cellular ionic balance under salt stress conditions (Yang and Guo, 2018). The undesirable impacts of excess salinity on plants are possibly attributed to osmotic stress, cytotoxicity provoked by excess Na+ and Cl-, nutritional imbalances, and decreased turgor (Kaur et al., 2022; Farouk and AL-Huqail, 2022).
Salt stress also causes ionic imbalances, the osmotic effect, water use deficiency, and nutrient deficiency (e.g., N, Ca, K, P, Fe, and Zn), which ultimately leads to oxidative stress in plants (Rehman et al., 2019). Silicon, which is the second most abundant element in soil, is well-known to increase plant tolerance to multiple abiotic stresses such as salinity (Thorne et al., 2020). Furthermore, Silicon can be described as a "multi-talented" element and can improve soil conditions and nutrient content (e.g., N, P, and K) in plants, making it a valuable fertilizer for promoting ecologically sound agricultural practices (Zargar et al., 2019). In several studies, foliar sprays of silicate (e.g., K2SiO3 or Na2SiO3), silica nanoparticles, and stabilized silicic acid have been shown to effectively increase plant growth (Laane, 2017). Foliar application of silicon (Si), including silicates, stabilized silicic acid, and silica nanoparticles, has been investigated in numerous studies. Similar to root application, foliar sprays with Si compounds effectively enhance growth and yield while mitigating biotic and abiotic stresses (Laane, 2018). However, the beneficial effects of foliar silicon on salt stress have only been experimentally tested in a few plant species. In cucumber, foliar spray of SiO2 nanofertilizers increased nitrogen (N) and phosphorus (P) content and uptake, and reduced sodium (Na+) content and uptake under salt stress (Yassen et al., 2017). Interestingly, Si's ability to alleviate various stresses has been confirmed even in species with low Si accumulation potential, such as tomatoes (Hoffmann et al., 2020). Silicon also enhances plant tolerance to a wide range of biotic and abiotic stresses, including drought, salinity, heat, cold, metal toxicity, and nutrient stress (Bhat et al., 2019; Hasanuzzaman et al., 2018). Recently, the advancements and mechanisms of Si in alleviating various biotic and abiotic stresses in plants have been systematically reviewed by several researchers (Coskun et al., 2019; Debona et al., 2017; Luyckx et al., 2017). In general, Si may improve salinity tolerance by regulating Na+ and K+ transport and accumulation, which are the primary salt tolerance mechanisms in plants (Zhu and Gong, 2014). Si has been shown to increase the concentration of macronutrients, such as Ca, P, and Mg, and micronutrients, such as B, Fe, Zn, and Mn, in a variety of plant species (Zhu and Gong, 2014). Si is known to reduce Na+ uptake by stimulating the activity of the root plasma membrane H+-ATPase (Xu et al., 2015) and to minimize Na+ translocation by enhancing its binding to cell walls, thus reducing Na+ in the leaf apoplast. Coskun et al. (2016) reported that silicon-mediated stimulation of proton pumps located on the plasma membrane and tonoplast increases Na+ efflux and K+ influx to plant cells. In previous studies indicated that Si treatment enhanced the uptake of nutrients such as K (Chen et al., 2016), Mg (Xu et al., 2015), Ca (Mateos-Naranjo et al., 2015), Fe (Pavlovic et al., 2013), Zn (Pascual et al., 2016), Mn (Wang and Han, 2007), and Cu (Gunes et al., 2008) in plants under drought and salt stress. Exogenous silicon application has been shown to mitigate oxidative stress in many plant species, including tomatoes (Shi et al., 2014), cucumber (Pavlovic et al., 2013), strawberry (Muneer et al., 2017), and rapeseed (Hasanuzzaman et al., 2018). There are limited research studies on the effects of Si on woody plant responses to salinity stress. Therefore, the purpose of the current study was to elucidate the effects of exogenous silicon on growth, compatible solutes, enzymatic antioxidants, and mineral nutrient content under salinity stress in two grapevine cultivars (Vitis vinifera L.).
Well-rooted cuttings of two grapevine cultivars, Vitis vinifera L. 'Bidaneh Ghermez' and 'Sahibi Gird', were transplanted into pots filled with a mixture of perlite and cocopeat (1:1 v/v) in an open hydroponic system. The pots were maintained in a greenhouse with a photoperiod of 16 hours of light and 8 hours of darkness, a relative humidity of 50 to 60%, and average minimum and maximum temperatures of 19 ± 3°C and 27 ± 3°C, respectively. The plants were fertigated with a modified ½ strength Hoagland nutrient solution consisting of 2.5 mM Ca (NO3), 1 mM MgSO4, 2.5 mM KNO3, 0.5 mM KH2PO4, 0.3 μM CuSO4, 6 μM MnSO4, 0.7 μM ZnSO4, 23 μM H3BO3, 32 μM Fe-EDTA, and 0.1 μM H2MoO4 (Hoagland and Arnon, 1950). The pH of the nutrient solution was maintained at 6.1. At the onset of the experiment, the plants were supplied with 150 mL of nutrient solution three times a week. The nutrient solution was renewed weekly.
Salinity stress was imposed on two-year-old grapevine plants fertilized with ½ strength Hoagland nutrient solutions. The final concentrations of NaCl salinity were 40 mM and 80 mM. Control plants received only nutrient solutions. The electrical conductivity (EC) of the salt-treated solutions at 25°C was 3.5 dsm-1 and 7.8 dsm-1, respectively. To minimize salinity shock, NaCl was gradually added to the nutrient solutions at 40 mM per day to achieve the desired salinity level. The experiment lasted six weeks.
Potassium silicate was applied to the foliage at rates of 0, 50, 100, and 200 mg L-1. A control group of plants was grown without NaCl and sprayed with deionized water. Tween-20 (0.1%) was added to all solutions as a surfactant. The plants were sprayed with potassium silicate solutions at two-week intervals throughout the experimental period.
After harvesting, the leaves, stems, and roots of the plants from each pot were separated. They were then washed, and their fresh weights were recorded immediately after drying using a digital scale (PJ300, METTLER) with 0.001 g precision. To determine the dry weight of leaf and root, the samples were first oven-dried at 70°C for 72 hours and then reweighed using a digital scale.
Proline content was determined following the method described by Irigoyen et al. (1992). Fresh leaf material (0.5 g) was homogenized in 5 mL of 75% ethanol, and the homogenate was centrifuged at 3,500 rpm. The alcoholic extract was stored in the refrigerator (4°C) until proline and soluble sugar measurements were conducted (Irigoyen et al., 1992). One milliliter of the supernatant was combined with 5 mL of acid ninhydrin and 5 mL of glacial acetic acid in a test tube. The mixture was placed in a water bath for 45 min at 100°C. The reaction mixture was extracted with 10 mL of benzene, and the homogenate was centrifuged. The supernatant was cooled to room temperature, and the absorbance was measured at 515 nm using a UV/visible spectrophotometer. Appropriate proline standards were included to calculate the proline content of the samples (Paquin and Lechasseur, 1979). For soluble sugar quantification, 0.1 mL of the alcoholic extract stored in the refrigerator was added to a test tube using a micropipette and 3 mL of Anthon reagent (150 mg Anthon + 100 mL of 72% w/w sulfuric acid). The test tubes were placed in boiling water for 10 min. After cooling the samples, their absorption rate was determined at a wavelength of 625 nm using a spectrophotometer (Irigoyen et al., 1992).
Mature leaves were utilized for protein content determination. Leaf segments were ground in cold phosphate buffer (pH 6.5) and filtered. The filtrate was centrifuged at 4,000 g for 20 minutes at 4°C. The supernatant was decanted, and then the Bradford Protein Kit was added. The mixture was vigorously shaken with a vortex. The sample absorbance was determined at 595 nm. The protein levels were estimated according to the Bradford (1976) method using bovine serum albumin as a standard and expressed in milligrams of protein per gram of fresh weight (FW).
Leaves were sampled for the determination of antioxidant enzyme activities: catalase (CAT), Guaiacol Peroxidase (GPX), and Ascorbate peroxidase (APX). Plant extracts were prepared to assess enzymatic activity (Kang and Saltveit, 2002). Fresh leaf samples (0.5 g) were homogenized at 0-4°C in 3 mL of 50 mM Tris buffer (pH 7.8), containing 1 mM EDTA-Na2 and 7.5% polyvinylpyrrolidone. Leaf samples were centrifuged at 12,000 rpm for 20 minutes, and the antioxidant enzyme activities were assayed in the supernatant using a spectrophotometer.
Catalase (CAT) activity was determined according to the method described by Aebi (1984). The CAT reaction mixture included 2.5 mL of 50 mM phosphate buffer (pH = 7) and 0.2 mL of hydrogen peroxide (H2O2) at a concentration of 0.1%. This mixture was rapidly mixed with 0.3 mL of enzyme extract. Changes in absorbance at 240 nm were measured after 1 min using a spectrophotometer. The catalase activity was calculated using the following formula:
Guaiacol peroxidase (GPX) activity was determined using the method described by Updhyaya et al. (1985). The GPX reaction mixture included 2.5 mL of 50 mM phosphate buffer (pH = 7), 1 mL of guaiacol (1%), and 1 mL of hydrogen peroxide (H2O2). This mixture was rapidly mixed with 0.1 mL of enzyme extract. The light absorbance of the reaction solution was measured after 1 min at 420 nm. The guaiacol peroxidase activity was calculated using the following formula:
Ascorbate peroxidase (APX) activity was determined following the method described by Nakano and Asada (1981). The AXP reaction mixture consisted of 2.5 mL of 50 mM phosphate buffer (pH = 7), 0.1 mL of EDTA, 1 mM sodium ascorbate, and 0.2 mL of hydrogen peroxide (H2O2). This mixture was rapidly mixed with 0.1 mL of enzyme extract. The absorbance of the reaction solution was measured after 1 min at 290 nm. The APX activity was calculated using the following formula:
Upon completion of the experiment, mature leaves from the mid-stem were harvested and oven-dried at 70°C for 48 hours. The dried tissues were subjected to analysis to determine their concentrations of NO3-N, phosphorus, potassium, sodium, zinc, iron, and manganese. Sodium and potassium levels in the leaves were assessed via flame photometry (Fater 405 model, Iran) (Mizukoshi et al., 1994). Nitrogen as nitrate (NO3-N) was determined colorimetrically through the nitration of salicylic acid (Cataldo et al., 1975). Phosphorus content was estimated using the colorimetric molybdenum vanadate method, employing a spectrophotometer (UV-2100) at 470 nm (Ohyama, 1991). Another set of samples was prepared to determine the concentrations of manganese, iron, and zinc. Dried samples (0.4 g) were individually ground and ashed in a porcelain crucible at 550°C for 5 hours. The white ash was subjected to digestion using 10 mL of 2 M hydrochloric acid (HCl). The digested solution was then filtered into a 50 mL volumetric flask and brought to a final volume of 50 mL with distilled water. The concentrations of manganese, iron, and zinc were determined using an atomic absorption spectrophotometer (Shimadzu AA-6300, Japan) (Ghazan Shahi, 1997).
The experiments were conducted as factorial trials utilizing a completely randomized design. Data analysis was performed using analysis of variance (ANOVA) in SAS v. 9.1 Statistical Software. Significant differences among the means were established at p < 0.05.
Increasing NaCl levels in the nutrient solution resulted in a decline in both fresh and dry leaf weights. The highest fresh weight (4.98 g) and dry leaf weight (2.4 g) were observed in the treatment with 200 mg L-1 potassium silicate and zero salinity (Fig. 1). As NaCl levels increased, both 'Bidaneh Ghermez' and 'Sahibi Gird' cultivars exhibited reduced fresh and dry root weights. In response to 40 mM salinity treatment, the rate of root fresh weight loss was reduced by 18.2%, 9%, and 7.2% for treatments with 50, 100, and 200 mg L-1 potassium silicate, respectively, compared to the control. Without potassium silicate application, the rate of root dry weight loss was 47.44% and 58.32% for 40 mM and 80 mM NaCl treatments, respectively. However, with potassium silicate application at 200 mg L-1, the rate of root dry weight loss was reduced to 28% and 66.4% for 40 mM and 80 mM NaCl treatments, respectively. The application of potassium silicate at 80 mM NaCl level did not effectively mitigate the adverse effects of salinity on root dry weight (Fig. 1).
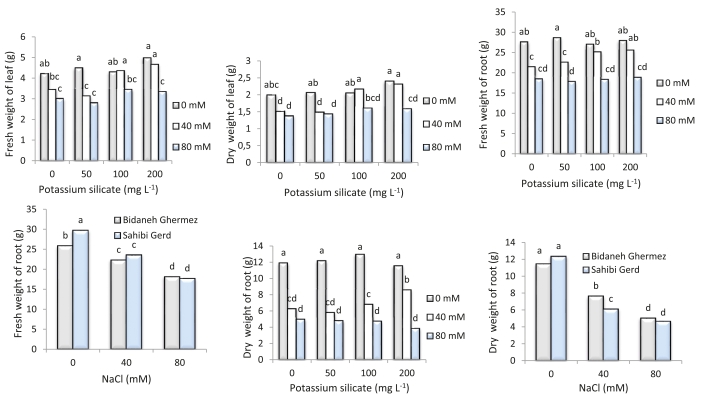
Fig. 1: Interaction effects of potassium silicate and NaCl on leaf and root fresh and dry weight in two grapevine cultivars ('Bidaneh Ghermez' and 'Sahibi Gird'). Different letters indicate a significant difference (p<0.05) between different treatments.
Increasing NaCl levels resulted in elevated proline levels in both cultivars. The peak proline concentration was observed in the treatment with 80 mM salinity (317.13 mg g-1 of fresh weight) and potassium silicate at 50 mg L-1 (Fig. 2).
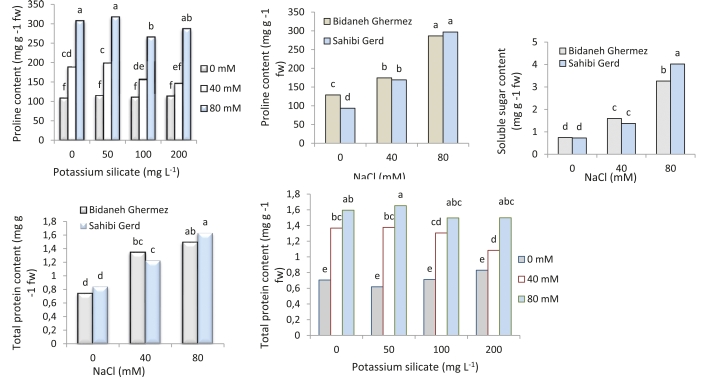
Fig. 2: Interaction effects of potassium silicate and NaCl on proline levels, soluble sugars content, and total protein content in two grapevine cultivars ('Bidaneh Ghermez' and 'Sahibi Gird'). Different letters indicate a significant difference (p<0.05) between different treatments.
Increased NaCl levels led to a substantial increase in soluble sugars content in both 'Bidaneh Ghermez' and 'Sahibi Gird' cultivars, with a 4.4-fold and 54.5-fold increase, respectively, compared to the control (Fig. 2).
Total protein content in both cultivars exhibited an increasing trend with elevated NaCl levels. This upward trajectory was observed at the 80 mM salinity level for 'Bidaneh Ghermez' (1.49 mg L-1 fresh weight) and 'Sahibi Gird' (1.63 mg L-1 fresh weight). The maximum total protein content (1.65 mg L-1 fresh weight) was detected at the 80 mM NaCl level and potassium silicate application at 50 mg L-1 (Fig. 2).
With increasing NaCl concentrations in the external environment, the activity of ascorbate peroxidase (APX) in both 'Bidaneh Ghermez' and 'Sahibi Gird' increased by 3.53-fold and 4.7-fold, respectively, compared to the control (Fig. 3). The maximum APX activity (7.54 μmol min-1 fresh weight) was observed at a potassium silicate concentration of 50 mg L-1 (Fig. 3).
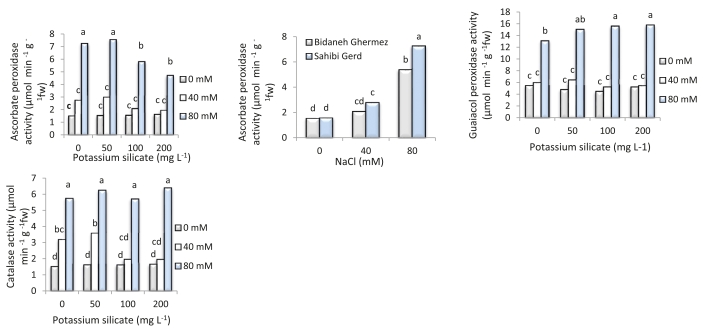
Fig. 3: Interaction effects of potassium silicate and NaCl on activity of ascorbate peroxidase, guaiacol peroxidase, and catalase enzymes in two grapevine cultivars ('Bidaneh Ghermez' and 'Sahibi Gird'). Different letters indicate a significant difference (p<0.05) between different treatments.
At an external NaCl of 80 mM, the application of potassium silicate at concentrations of 50, 100, and 200 mg L-1 resulted in a 2.76, 2.87, and 2.9-fold increase in GPX activity, respectively, compared to the control plants (Fig. 3).
In 50, 100, and 200 mg L-1 potassium silicate treatments, catalase enzyme activity in the 80 mM NaCl treatment was 4.19, 3.82, and 4.28-fold higher than the control treatment, respectively (Fig. 3).
As NaCl levels in the nutrient solution increased, the amount of nitrate in the leaves decreased. In treatments with 40 and 80 mM NaCl (without potassium silicate), nitrate levels decreased by 32.34% and 46.71%, respectively, compared to the control group (Fig. 4). When potassium silicate was applied at concentrations of 100 and 200 mg L-1 in the 40 mM NaCl treatment, the rate of nitrate loss was 22.16% and 17.37%, respectively, compared to the control group.
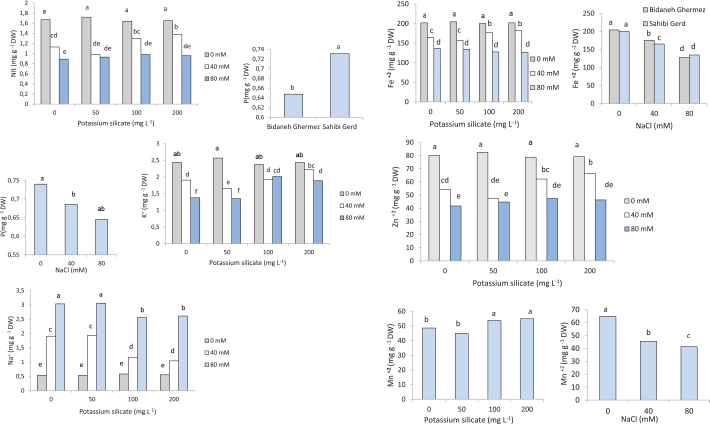
Fig. 4: Interaction of potassium silicate and NaCl on nitrate, phosphorus, potassium, sodium, iron, zinc, and manganese content in two grapevine cultivars ('Bidaneh Ghermez' and 'Sahibi Gird'). Different letters indicate a significant difference (p<0.05) between different treatments.
As NaCl levels increased, the amount of leaf phosphorus decreased. At 40 mM NaCl, phosphorus content was 7.43% lower than the control, and at 80 mM NaCl, it was 12.84% lower (Fig. 4). Phosphorus content in 'Bidaneh Ghermez' was 0.65% and in 'Sahibi Gird', 0.73%.
The presence of sodium chloride (NaCl) in the root medium caused a decrease in potassium (K+) content in the leaves of both cultivars. However, the application of potassium silicate at a concentration of 200 mg L-1 mitigated this reduction in potassium levels. At 40 mM NaCl, potassium levels were reduced by 9.02% with potassium silicate application, compared to the control without potassium silicate. At 80 mM salinity, the reduction in potassium levels with potassium silicate application was 22.54% compared to the control (Fig. 4).
In both cultivars, the sodium (Na+) content in leaves increased along with the increase in NaCl concentration in the nutrient solution. The highest Na+ concentration was observed in the 80 mM NaCl treatment, regardless of whether potassium silicate was applied or not at a concentration of 50 mg L-1. Potassium silicate treatment significantly reduced Na+ concentration in leaves of both cultivars under NaCl stress conditions (Fig. 4).
The lowest iron content in both cultivars was observed in the 80 mM NaCl treatment. In this treatment, leaf iron concentration in 'Bidaneh Ghermez' and 'Sahibi Gird' cultivars decreased 1.60 and 1.48 times, respectively, compared to the control. The amount of leaf iron in the 40 mM NaCl treatment increased by 7.64% with potassium silicate at a concentration of 100 mg L-1 and by 10.47% with potassium silicate at a concentration of 200 mg L-1 (Fig. 4).
In the 40 mM NaCl treatment, zinc content with the application of potassium silicate at concentrations of 100 and 200 mg L-1 showed an increase of 14.35% and 22.4%, respectively (Fig. 4).
With increasing NaCl concentration in the nutrient solution, manganese (Mn) concentration decreased by 29.7% in the 40 mM salinity treatment and by 36.1% in the 80 mM NaCl treatment. With increasing the concentration of potassium silicate, the amount of Mn increased, so that in the treatments with 100 and 200 mg L-1 of potassium silicate, this increase was 10.6% and 13.2%, respectively, compared to the control (Fig. 4).
Salinity stress induces a diverse array of responses in plants, leading to morphological, physiological, biochemical, and molecular alterations. It can also impair physiological and biochemical processes such as photosynthesis, protein synthesis, transpiration, and lipid metabolism (Muchate et al., 2016). Salinity has been shown to reduce dry root weight in both cultivars. This study revealed a negative correlation between salinity stress and vegetative growth parameters, including fresh and dry weight of leaves, shoots, and roots. The most detrimental effect of salinity on vegetative traits was observed at the highest NaCl level (80 mM), though this effect was evident in both cultivars. However, the responses of the two cultivars differed. In plants subjected to salinity stress, NaCl ions accumulate in the roots of both salt-tolerant and salt-sensitive crops. High salinity levels can significantly hinder plant growth and development (Lei et al., 2015). High salt levels can lead to both water deficit and ion toxicity, adversely affecting various aspects of plant function, metabolism, and structure. In this study, the observed growth decline under salinity stress was attributed to nutritional imbalances and excessive sodium (Na+) uptake. These findings align with previous studies on the detrimental effects of salinity on plant growth. Roots are the primary organs directly exposed to salinity stress, experiencing a more severe growth reduction than other plant tissues such as shoots. These findings are consistent with previous reports (Aazami et al., 2023; El-Banna et al., 2022; Liu et al., 2020).
In recent years, research has focused on understanding the role of silicon (Si) in mitigating abiotic and biotic stresses, particularly drought and salinity, the two most prevalent stress factors. Si has been demonstrated to regulate root growth in salt-stressed plants (Zhu et al., 2015). Applying silicon (Si) as a foliar spray mitigated the growth inhibition caused by added sodium chloride (NaCl). In cucumber, Si was found to increase the root-to-shoot ratio of salt-stressed plants and improve root hydraulic conductance, potentially contributing to enhanced plant water balance (Wang et al., 2015). In rice and sorghum, Si may improve root growth by increasing casparian band formation, stimulating suberin and lignin biosynthesis, or promoting cell wall extensibility in the growth region (Fleck et al., 2015). With increasing salinity levels, the levels of proline, an osmoprotectant, increased in both grapevine cultivars. The highest proline levels were observed at the highest salinity level (80 mM) and with the addition of 50 mg L-1 potassium silicate (317.13 mg g-1 fresh weight). This study demonstrated that both moderate (40 mM) and high (80 mM) salinity induced a significant increase in free proline content in the leaves of both grapevine cultivars. Proline synthesis is considered an adaptive mechanism to counteract salt stress. Osmolytes like proline help stabilize functional proteins, enzymes, protein complexes, and cell membranes under salt stress conditions (Rajasheker et al., 2019). In this study, the enhancement in proline levels likely resulted from a combination of its osmotic and antioxidant properties. Similar observations have been reported in other crops, including Brassica juncea (Khan et al. 2012), Linum usitatissimum (Nasir Khan et al. 2009), Morus alba (Ahmad et al. 2013), and Cicer arietinum (Ahmad et al., 2016).
Our results demonstrate that supplemental silicon (Si) enhanced proline accumulation in grapevine leaves exposed to salt stress. Si plays a crucial role in modulating the expression of silicon transporters (Lsi1 and Lsi2) and stress-related proteins, leading to increased silica accumulation and elevated levels of compatible solutes in plants fertilized with Si (Thorne et al., 2020). Additionally, the elevated proline levels in the tissues may have further contributed to this effect by protecting cellular structures. Therefore, it is reasonable to conclude that Si improved the antioxidant capacity of grapevine plants under salt stress. In this experiment, we observed an increase in soluble sugars under stress, which aligns with the findings of other researchers studying various plant species. Numerous studies have reported that Si application can enhance plant tolerance to salinity stress by altering the levels of solutes such as carbohydrates (Ming et al., 2012), proline (Yin et al., 2013), glycine betaine (Torabi et al., 2015), polyols, and antioxidant compounds like total phenolics (Hashemi et al., 2010). These alterations help mitigate the osmotic shock induced by NaCl stress due to ion toxicity (Na+ and Cl-). In cucumber, treatment with Na + Na2SiO3_9H2O was found to increase the accumulation of soluble sugars (primarily sucrose and glucose) and reduce the osmotic potential of xylem sap in the root system compared with Na+ treatment. This effect contributed to enhanced root water uptake (Zhu et al., 2016).
As salinity levels increased, soluble protein content rose in both grapevine cultivars. Ali et al. (2013) observed that soluble protein content was alleviated by salinity and significantly increased in the presence of Si in sunflower. Al-Aghabary et al. (2004) proposed that Si acts as a promoter of protein synthesis, as the presence of this nutrient in salt-stressed plants enhances the synthesis of mRNA related to the synthesis of nitrogenous compounds. These findings suggest that grapevine plants similarly responded to Si, as evidenced by the increased soluble protein content in leaves of plants provided with Si. Salinity tolerance is contingent on an effective antioxidant system. In this study, as salinity increased, the activity of antioxidant enzymes followed an upward trend. To minimize ROS-induced damage to biomolecules, plants should efficiently utilize their antioxidant defense system, which consists of both enzymatic and non-enzymatic antioxidants (Hasanuzzaman et al., 2019).
The present study demonstrates that supplemental silicon enhanced the activity of antioxidant enzymes in both grapevine cultivars. Hasanuzzaman et al. (2018) reported that exogenous Si application (1 mM) increased the activity of APX, MDHAR, GR, GST, DHAR, CAT, and GPX and boosted AsA and GSH levels in Brassica napus. These findings corroborate our results, suggesting that Si can significantly augment the antioxidant capacity of grapevine plants under salt stress. Similar observations have been reported in other plant species, such as grapevine (Soylemezoglu et al., 2009), tomato (Li et al., 2015), and okra (Abbas et al., 2015). These studies collectively indicate that Si has the potential to induce a robust antioxidant defense system that effectively counteracts reactive oxygen species (ROS) buildup and protects grapevine plants from salt stress-induced damage. Salt stress can exacerbate nutritional deficiencies in plants (Gupta and Huang, 2014). High levels of salts can lead to nutrient imbalances, as excessive sodium (Na+) uptake can interfere with the uptake of essential nutrients.
This is because the hydrated ionic radii of K+ and Na+ are similar, making it challenging for the cell membrane transport system to distinguish between these two ions. This misidentification can cause Na+ ions to be transported into cells via K+ transporters, leading to Na+ toxicity under high salinity conditions (Blumwald, 2000). Na+ toxicity, particularly at salinity levels exceeding 40 mM, has been observed in both grapevine cultivars. Plants have developed various mechanisms to cope with salt stress, including ion-uptake regulation, vacuolar compartmentation, and ion exclusion. These strategies help maintain cellular ion balance and protect plants from the harmful effects of salinity (Blumwald, 2000). However, excessive accumulation of Na+ and Cl− ions during saline conditions can still hinder the uptake of essential nutrients (Ahanger and Agarwal, 2017).
When Na+ concentration is elevated, K+ uptake is often inhibited, leading to increased Na+ accumulation in plant tissues. K+ ions enter the cell through a series of ion channels located on the cell membrane, and these channels can easily be used for the penetration of Na+ ions into the cell under salt stress conditions (Mita et al., 2021). Supplemental Si can effectively block the uptake of Na+ ions and act as a physical barrier to their root-to-shoot translocation through the apoplastic bypass route (Yeo et al., 1999). This mechanism effectively prevents Na+ accumulation in the shoots. Yan et al. (2021) investigated the role of Si in regulating Na+ transport and its movement from roots to shoots in rice. They found that Si supplementation increased lignification and suberization of the casparian band, the barrier between the root cortex and stele. These structural modifications, driven by changes in gene expression related to phenol biosynthesis (Hinrichs et al., 2017), further protect plants from uncontrolled Na+ influx. This research suggests that Si can mitigate the negative effects of salinity by preventing Na+ uptake by the roots and its subsequent movement to the shoots. Silicon protective effects are likely due to its ability to form a physical barrier, enhance lignification and suberization, and modulate gene expression to regulate Na+ transport. Silicate, particularly potassium silicate, acts as a protective barrier against salt stress by preventing Na+ translocation from roots to aerial parts of the plant. It achieves this by either depositing Na+ in epidermal cells, forming a physical barrier to ion movement, or forming complexes with freely available Na+ and Si ions. A recent study by Bosnic et al. (2018) demonstrated that Si supplementation to moderately NaCl-stressed maize plants effectively reduced Na+ concentration in the root symplast by up-regulating the expression of SOS1 (responsible for Na+ efflux) and down-regulating the expression of HKT1 (responsible for Na+ influx). This mechanism helps prevent Na+ accumulation in the plant, protecting it from the detrimental effects of salt stress.
In grapevines, the application of potassium silicate resulted in a significant increase in Na+ exclusion, as evidenced by reduced Na+ levels in leaves even at elevated salinity levels. This protective effect is likely due to Si's ability to form a physical barrier, enhance lignification and suberization of the casparian band, and modulate gene expression to regulate Na+ transport. A sharp decline in potassium ion (K+) content was observed in the leaves of both grape cultivars as salt concentration increased. It is now understood that K+ ions can enter cells through channels that are often more permeable to sodium ions (Na+) under saline conditions (Parida and Das, 2005). Due to the physicochemical similarity between Na+ and K+ (e.g., ionic radius and ion hydration energy), Na + competes with K+ for different binding sites in crucial metabolic processes within the cytoplasm (Shabala and Cuin, 2008). This suggests competition between Na + and K+ in grapevines. The depletion in K+ uptake caused by Na+ is likely to stem from the competitive influx of both ions into the cells. There are limited studies on the impact of silicon (Si) on enhancing K+ ion uptake under salinity stress. However, silicon has been shown to improve K+ nutrition under salt stress in various plant species (Rizwan et al., 2015; Coskun et al., 2016; Zhu et al., 2019a).
A study by Liang (1999) demonstrated that supplemental Si can improve K+ uptake in salt-stressed barley by ameliorating K+ selectivity. In line with these findings, potassium silicate alleviated the detrimental effects of Na+ in grapevine leaves by boosting K+ content. The present study revealed a significant decline in NO3-N content in salt-stressed grapevines. This reduction indicates that salinity disrupts ionic homeostasis in grapevines and negatively impacts N nutrition. The application of potassium silicate alleviated these disturbances by enhancing NO3-N uptake, assimilation, and remobilization (Gou et al., 2020). Haddad et al. (2018) reported that Si supplementation increased N uptake in Brassica napus plants, accompanied by an upsurge in the expression of a nitrate transporter gene in roots. Our findings corroborate these observations, as potassium silicate treatment resulted in elevated NO3-N content in grapevine leaves. Along with NO3-N, salinity also reduced leaf phosphorus content in both cultivars. At the highest salinity level of 80 mM, phosphorus content decreased by 12.84% relative to the control. Phosphorus deficiency is known to hinder plant growth and biomass production (Sulieman and Tran, 2015). However, potassium silicate treatments effectively counteracted this deficiency by increasing phosphorus concentration in grapevine leaves. Two primary mechanisms have been proposed to explain Si's ability to alleviate phosphorus deficiency: 1. Enhanced root uptake: Si can increase the expression of phosphorus transporters in root cells, leading to improved phosphorus uptake from the soil. 2. Enhanced utilization of phosphorus within plant tissues: Si may improve the efficiency of phosphorus utilization by enhancing phosphorus partitioning and metabolism.
Studies have demonstrated increased phosphorus uptake following Si fertilization in various crops (Neu et al., 2017; Zhang et al., 2019). These findings suggest that potassium silicate can be a valuable tool for mitigating the negative effects of salinity stress on grapevines by improving nitrogen and phosphorus nutrition. Silicon can enhance phosphorus availability in soil by modifying soil pH, reducing phosphorus absorption by soil minerals due to competition between phosphorus and silicon (depending on silicon speciation in soil solution) (Schaller et al., 2021). With increasing salinity in the nutrient solution, iron (Fe) content in the leaves decreased. The lowest Fe amount was observed in both cultivars at 80 mM NaCl. In this treatment, leaf Fe concentration in 'Bidaneh Ghermez' and 'Sahibi Gird' cultivars decreased by 1.60 and 1.48 times, respectively, compared to the control. In cucumber, exogenous silicon application alleviates iron deficiency by coordinating the expression of genes involved in iron acquisition (Pavlovic et al., 2013). The effect of silicon on iron nutrition has been demonstrated in various plant species grown under optimal, low, or high iron conditions (Becker et al., 2020; Hernández-Apaolaza et al., 2020).
Additionally, Stevic et al. (2016) showed that the addition of Si (OH)4 to iron-deprived cucumber plants could enhance iron bioavailability by forming an iron-silicon complex and maintaining the redox potential in both root apoplastic and xylem fluids, thus facilitating iron translocation from roots to shoots via the xylem. Furthermore, Nikolić et al. (2019) recently reported that silicon alleviates iron deficiency in barley (a Strategy 2 species) by amplifying the expression of genes involved in iron uptake and transport in roots. In both cultivars, the amount of zinc (Zn2+) and manganese (Mn2+) decreased with increasing salinity levels in the nutrient solution. Potassium silicate application, particularly at concentrations of 100 and 200 mg L-1, significantly boosted Zn2+ content in grapevine leaves. Pascual et al. (2016) proposed that Si treatment enhances Zn2+ accumulation in the root apoplast and its movement to shoots when soybean plants are subjected to Zn2+ deficiency, thereby alleviating stress symptoms. For instance, Si application has been shown to mitigate certain symptoms of Zn-deficiency in cucumber plants, most likely due to its indirect effect of enhancing the antioxidant defense capacity in plant tissues, rather than its direct effect on Zn mobility, uptake, and tissue distribution (Bityutskii et al., 2014). Potassium silicate treatments effectively increased Mn2+ concentration in leaves of both grapevine cultivars. These findings are consistent with those of Greger et al. (2018), who demonstrated that Si application to soil increases Mn2+ availability and improves Mn2+ uptake and translocation to shoots in various plant species grown under conditions of adequate Mn2+ supply.
Salinity stress encompasses intricate and multifaceted mechanisms that are linked to distinct metabolic pathways in various plant organs. Plants respond to high-salinity stress by employing a variety of mechanisms, including the modulation of Na+ uptake and translocation, activation of their antioxidant defense system, accumulation of compatible solutes, and osmotic regulation. These responses play a crucial role in plant adaptation to salt stress. Competition between Na+ ions and K+ ions, a consequence of salinity stress, impedes plant growth by disrupting nutrient accessibility. However, application of silicon (Si) mitigates these stress-induced detrimental effects by regulating various physiological and biochemical processes, such as Na+ balance, enhancing the activity of antioxidant enzymes (CAT, APX, and GPX), and accumulation of compatible solutes, in both grapevine cultivars. In conclusion, the results of this study suggest that application of potassium silicate can be used as an effective strategy to reduce the negative effects of salinity stress on grapevines.
The authors declare that they do not have any conflicts of interest.
Aazami, M. A., Maleki, M., Rasouli, F., Gohari, G., 2023: Protective effects of chitosan based salicylic acid nanocomposite (CS‑SA NCs) in grape (Vitis vinifera cv. ‘Sultana’) under salinity stress. Scientific Reports 13, 883, DOI: 10.1038/s41598-023-27618-z.
Abbas, T., Balal, R. M., Shahid, M. A., Pervez, M. A., Ayyub, C. M., Aqueel, M. A., Javaid, M. M., 2015: Silicon-induced alleviation of NaCl toxicity in okra (Abelmoschus esculentus) is associated with enhanced photosynthesis, osmoprotectants and antioxidant metabolism. Acta Physiologiae Plantarum 37, 1–15, DOI:10.1007/s11738-014-1768-5.
Aebi, H., 1984: Catalase in vitro. In Methods in Enzymology; Oxygen Radicals in Biological Systems; Academic Press: Cambridge, MA, USA, 105, 121–126.
Ahanger, M. A., Agarwal, R. M., 2017: Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L) as influenced by potassium supplementation. Plant Physiology and Biochemistry 115, 449–460, DOI: 10.1016/j.plaphy.2017.04.017.
Ahmad, P., Ozturk, M., Sharma, S., Gucel, S., 2013: Effect of sodium carbonate- induced salinity–alkalinity on some key osmoprotectants, protein profile, antioxidant enzymes, and lipid peroxidation in two mulberry (Morus alba L.) cultivars. Journal of Plant Interactions 9, 460–467, DOI: 10.1080/17429145.2013.855271.
Ahmad, P., Abdel Latef, A. A., Hashem, A., Abd_Allah, E. F., Gucel, S., Tran, L. S. P., 2016: Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Frontiers in Plant Science 7, DOI: 10.3389/fpls.2016.00347.
Al-Aghabary, K., Zhu, Z., Shi, Q. H., 2004: Influence of silicon supply on chlorophyll content, chlorophyll fluorescence, and antioxidative enzyme activities in tomato plants under salt stress. Journal of Plant Nutrition 27, 2101–2115, DOI: 10.1081/PLN-200034641.
Ali, M., A., Ramezani A, Far, S. M., Sadat, K., 2013: Application of silicon ameliorates salinity stress in sunflower (Helianthus annuus L.) plants. International Journal of Agriculture and Crop Sciences 6, 1367–1372.
Becker, M., Ngo, N. S., Schenk, M. K. A., 2020: Silicon reduces the iron uptake in rice and induces iron homeostasis related genes. Scientific Reports 10,5079, DOI: 10.1038/s41598-020-61718-4.
Bhat, J. A., Shivaraj, S. M., Singh, P., Navadagi, D. B., Tripathi, D. K., Dash, P. K., Solanke, A. U., Sonah, H., Deshmukh, R., 2019: Role of silicon in mitigation of heavy metal stresses in crop plants. Plants 8(3), 71, DOI: 10.3390/plants8030071.
Bityutskii, N., Pavlovic, J., Yakkonen, K., Maksimović, V., Nikolic, M., 2014: Contrasting effect of silicon on iron, zinc and manganese status and accumulation of metal-mobilizing compounds in micronutrient-deficient cucumber. Plant Physiology and Biochemistry 74,205-11, DOI: 10.1016/j.plaphy.2013.11.015.
Blumwald, E. 2000: Sodium transport and salt tolerance in plants. Current Opinion in Cell Biology, 12, 431-434, DOI: 10.1016/s0955-0674(00)00112-5.
Bosnic, P., Bosnic, D., Jasnic, J., Nikolic, M., 2018: Silicon mediates sodium transport and partitioning in maize under moderate salt stress. Environmental and Experimental Botany 155, 681–687, DOI: 10.1016/j.envexpbot.2018.08.018.
Bradford, M. M., 1976: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72(1-2), 248-254, DOI: 10.1006/abio.1976.9999.
Cataldo, D. A. M., Haroon, L. E., Schrader and Young, V. L., 1975: Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Soil Science and Plant Analysis 6, 71-80, DOI: 10.1080/00103627509366547.
Chen, D., Cao, B., Qi, L., Yin, L., Wang, S., Deng, X., 2016: Silicon-moderated K-deficiency-induced leaf chlorosis by decreasing putrescine accumulation in sorghum. Annals of Botany 118, 305–315, DOI: 10.1093/aob/mcw111.
Coskun, D., Britto, D. T., Huynh, W. Q., Kronzucker, H. J., 2016: The role of silicon in higher plants under salinity and drought stress. Frontiers in Plant Science 7, 1072, DOI: 10.3389/fpls.2016.01072.
Coskun, D., Deshmukh, R., Sonah, H., Menzies, J. G., Reynolds, O., Ma, J. F., Kronzucker, H., Bélanger, R.R., 2019: The controversies of silicon’s role in plant biology. New Phytologist 221, 67–85, DOI: 10.1111/nph.15343.
Cramer, G. R., Ergul, A., Grimplet, J., Tillett, R. L., Tattersall, E. A. R., Bohlman, M. C., Vincent, D., Sondergger, J., EVANS, J., Osborn, C., Quilice, D., Schlauch, K. A., Schooley, D. A., Cushman, J. C., 2007: Water and salinity stress in grapevines: early and late changes in transcript and metabolite profiles. Functional & Integrative Genomics 7, 111-134, DOI: 10.1007/s10142-006-0039-y. Epub 2006.
Cuena-Lombraña, A., Lallai, A., Belhadj, F., Gharbi, B., Bacchetta, G. 2022: Carignan grape cultivar salt tolerance during the germination phase across the mediterranean basin. Seeds 1, 136–145, DOI: 10.3390/seeds1020012.
Debona, D., Rodrigues, F. A., Datno, L. E., 2017: Silicon’s role in abiotic and biotic plant stresses. Annual Review of Phytopathology 55, 85–107, DOI: 10.1146/annurev-phyto-080516-035312.
El-Banna, M. F., AL-Huqail, A. A., Farouk, S., Belal, B. E. A., El-Kenawy, M. A., Abd El-Khalek, A. F., 2022: Morpho-physiological and anatomical alterations of salt-affected thompson seedless grapevine (Vitis vinifera L.) to brassinolide spraying. Horticulture, 8, 568. DOI: 10.3390/horticulturae8070568.
Farouk, S., AL-Huqail, A. A., 2022: Sustainable biochar and/or melatonin improve salinity tolerance in borage plants by modulating osmotic adjustment, antioxidants, and ion homeostasis. Plants 11(6), 765, DOI: 10.3390/plants11060765.
Fleck, A. T., Schulze, S., Hinrichs, M., Specht, A., Waßmann, F., Schreiber, L., Schenk, M. K., 2015: Silicon promotes exodermal Casparian band formation in Si-accumulating and Si-excluding species by forming phenol complexes. PLoS ONE 10, e0138555, DOI: 10.1371/journal.pone.0138555.
Ghazan shahi, J., 1997: Plant and Soil Analysis. Tehran, Iran: Homa Publications. 311 p.
Gou, T., Yang, L., Hu, W., Chen, X., Zhu, Y., Guo, J., Gong, H., 2020: Silicon improves the growth of cucumber under excess nitrate stress by enhancing nitrogen assimilation and chlorophyll synthesis. Plant Physiology and Biochemistry 152, 53–61, DOI: 10.1016/j.plaphy.2020.04.031.
Greger, M. Landberg, T. Vaculík, M. 2018: Silicon influences soil availability and accumulation of mineral nutrients in various plant species. Plants 7:41, DOI: 10.3390/plants7020041.
Grigore, M., Vicente, O., 2023: Wild Halophytes: Tools for Understanding Salt Tolerance Mechanisms of Plants and for Adapting Agriculture to Climate Change. Plants 12, 221, DOI: 10.3390/plants12020221.
Gunes, A., Pilbeam, D. J., Inal, A., Coban, S., 2008: Influence of silicon on sunflower cultivars under drought stress 1: growth antioxidant mechanisms and lipid peroxidation. Communications in Soil Science and Plant Analysis 39, 1885–1903, DOI: 10.1080/00103620802134651.
Gupta, B., Huang, B. R., 2014: Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. International Journal of Genomics 6, 727–740, DOI: 10.1155/2014/701596.
Haddad, C., Arkoun, M., Jamois, F., Schwarzenberg, A., Yvin, J. C., Etienne, P., Laîné, P., 2018: Silicon promotes growth of Brassica napus L. and delays leaf senescence induced by nitrogen starvation. Frontiers in Plant Science 9, 516, DOI: 10.3389/fpls.2018.00516.
Hasanuzzaman, M., Bhuyan, M., Anee, T. I., Parvin, K., Nahar, K., Mahmud, J. A., Fujita, M., 2019: Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants (Basel), 8(9), 384, DOI: 10.3390/antiox8090384.
Hasanuzzaman, M., Nahar, K., Rohman, M. M., Anee, T. I., Huang, Y., Fujita, M., 2018: Exogenous silicon protects Brassica napus plants from salinity-induced oxidative stress through the modulation of AsA-GSH pathway, thiol-dependent Aantioxidant enzymes and glyoxalase systems. Gesunde Pflanzen 70, 185–194, DOI: 10.1007/s10343-018-0430-3.
Hashemi, A., Abdolzadeh, A., Sadeghipour, H. R., 2010: Beneficial effects of silicon nutrition in alleviating salinity stress in hydroponically grown canola, Brassica napus L., plants. Journal of Soil Science and Plant Nutrition, 56, 244–253, DOI: 10.1111/j.1747-0765.2009.00443.x.
Hernández-Apaolaza, L., Escribano, L., Zamarreno, A. M., Garcia-Mina, J. M., Cano, C., Carrasco-Gil, S., 2020: Root silicon addition induces Fe deficiency in cucumber plants, but facilitates their recovery after Fe resupply. A comparison with si foliar sprays. Frontiers in Plant Science 11, 580552, DOI: 10.3389/fpls.2020.580552.
Hinrichs, M., Fleck, A. T., Biedermann, E., Ngo, N. S., Schreiber, L., Schenk, M. K., 2017: An ABC transporter is involved in the silicon-induced formation of Casparian bands in the exodermis of rice. Frontiers in Plant Science 8, 671, DOI: 10.3389/fpls.2017.00671.
Hoagland, D. R., Arnon, D. I., 1950: The water culture method for growing plants without soil. Management-Californiya Agriculture. 347.
Hoffmann, J., Berni, R., Hausman, J. F., Guerriero, G., 2020: A review on the beneficial role of silicon against salinity in non-accumulator crops: tomato as a model. Biomolecules 10, 1284, DOI: 10.3390/biom10091284.
Irigoyen, J. J., Emerich, D. W., Sanchez-Diaz, M., 1992: Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Plant Physiology 84, 55- 60, DOI: 10.1111/j.1399-3054.1992.tb08764.x.
Kang, H. M., Saltveit, M. E., 2002: Chilling tolerance of maize, cucumber and rice seedling leaves and roots and differentially affected by salicylic acid. Plant Physiology 115, 571-576, DOI: 10.1034/j.1399-3054.2002.1150411.x.
Kaur, H., Hussain, S.J., Kaur, G., Poor, P., Alamri, S., Siddiqui, M. H., Khan, I. M. R., 2022: Salicylic acid improves nitrogen fixation, growth, yield and antioxidant defense mechanisms in chickpea genotypes under salt stress. Journal of Plant Growth Regulation 41, 2034–2047, DOI: 10.1007/s00344-022-10592-7.
Laane, H., 2017: The effects of the application of foliar sprays with stabilized silicic acid: An overview of the results from 2003-2014. Silicon, 9, 803–807, DOI: 10.1007/s12633-016-9466-0.
Laane, H., 2018: The effects of foliar sprays with different silicon compounds. Plants, 7, 45, DOI: 10.3390/plants7020045.
Lei, X. G., Zhu, J. H., Cheng, W. H., Bao, Y., Ho, Y., Reddi, A. R., Holmgren, A., Arnér, E., 2015: Paradoxical roles of antioxidant enzymes: basic mechanisms and health implications. Physiological Reviews 96, 307–364, DOI: 10.1152/physrev.00010.2014.
Li, H., Zhu, Y., Hu, Y., Han, W., Gong, H., 2015: Beneficial effects of silicon in alleviating salinity stress of tomato seedlings grown under sand culture. Acta Physiologia Plantarum 37, 71, DOI: 10.1007/s11738-015-1818-7.
Liang, Y. C.; 1999: Effects of silicon on enzyme activity and sodium, potassium and calcium concentration in barley under salt stress. Plant Soil. 209 (2), 217–224, DOI: 10.1023/A:1004526604913.
Liu, C., Zhao, X., Yan, J., Yuan, Z., Gu, M., 2020: Effects of salt stress on growth, photosynthesis, and mineral nutrients of 18 pomegranate (Punica granatum) cultivars. Agronomy 10, 27, DOI: 10.3390/agronomy10010027.
Luyckx, M., Hausman, J.F., Lutts, S.; Guerriero, G., 2017: Silicon and plants: Current knowledge and technological perspectives. Frontiers in Plant Science 8, 411, DOI: 10.3389/fpls.2017.00411.
Mateos-Naranjo, E., Galle, A., Florez-Sarasa, I., Perdomo, J. A., Galmes, J., Ribas-Carbo, M., Flexas, J., 2015: Assessment of the role of silicon in the Cu-tolerance of the C4 grass Spartina densiflora. Journal of Plant Physiology 178, 74–83, DOI: 10.1016/j.jplph.2015.03.001.
Ming, D. F., Pei, Z. F., Naeem, M. S., Gong, H. J., Zhou, W. J., 2012: Silicon alleviates PEG-induced water-deficit stress in upland rice seedlings by enhancing osmotic adjustment. Journal of Agronomy and Crop Science 198,14–26, DOI: 10.1111/j.1439-037x.2011.00486.x.
Mita, K., Sumikama, T., Iwamoto, M., Oiki, S., 2021: Conductance selectivity of Na+ across the K+ channel via Na+ trapped in a tortuous trajectory. Proceedings of the National Academy of Sciences 118 (12), e2017168118, DOI: 10.1073/pnas.2017168118.
Mizukoshi, K., Nishiwaki, T., Ohtake, N., Minagawa, R., Kobayashi, K., Ikarashi, T., Ohyama, T., 1994: Determination of tungstate concentration in plant materials by HNO3-HClO4 digestion and colorimetric method using thiocyanate. Plant Analysis and Methods, 46, 51–6.
Muchate, N. S., Nikalje, G. C., Rajurkar, N. S., Suprasanna, P., Nikam, T. D., 2016: Plant salt stress: adaptive responses, tolerance mechanismand bioengineering for salt tolerance. Botanical Review 82, 371–406, DOI: 10.1007/s12229-016-9173-y.
Muneer, S., Park, Y. G., Kim, S., Jeong, B. R., 2017: Foliar or subirrigation silicon supply mitigates high temperature stress in strawberry by maintaining photosynthetic and stress-responsive proteins. Journal of Plant Growth Regulation 36, 836–845, DOI:10.1007/s00344-017-9687-5.
Nakano, Y., Asada, K., 1981: Hydrogen peroxide scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiology 22, 867–880, DOI: 10.1093/oxfordjournals.pcp.a076232.
Nasir Khan, M., Siddiqui M. H., Mohammad, F., Naeem, M., Khan, M. M. A., 2009: Calcium chloride and gibberellic acid protect linseed (Linum usitatissimum L.) from NaCl stress by inducing antioxidative defence system and osmoprotectant accumulation. Acta Physiologiae Plantarum 32, 121–132, DOI: 10.1007/s11738-009-0387-z.
Negrao, S., Schmöckel, S. M., Tester, M., 2017: Evaluating physiological responses of plants to salinity stress. Annals of Botany 119, 1–11, DOI: 10.1093/aob/mcw191.
Neu, S., Schaller, J., Dudel, E. G., 2017: Silicon availability modifies nutrient use efficiency and content, C: N: P stoichiometry, and productivity of winter wheat (Triticum aestivum L.). Scientific Reports 7,40829, DOI: 10.1038/srep40829.
Nikolic, D. B., Nesic, S., Bosnic, D., Kostic, L., Nikolic, M., Samardzic, J. T., 2019: Silicon alleviates iron deficiency in barley by enhancing expression of Strategy II genes and metal redistribution. Frontiers in Plant Science 10, 416, DOI: 10.3389/fpls.2019.00416.
Ohyama, T., 1991: Analytical procedures of N, P, K concentrations in plant and manure materials using H2SO4-H2O2 Kjeldahl digestion method. Japan. Bulletin Faculty of Agriculture, Niigata University, 43, 110-120.
Paquin, R., Lechasseur, P., 1979: Observations sur une method de dosage de la praline libre dans les extraits de plantes. Canadian Journal of Botany 57, 1851–1854.
Parida, A. K., Das, A. B., 2005: Salt tolerance and salinity effects on plants: A review. Ecotoxicology and Environmental Safety 60, 324–349, DOI: 10.1016/j.ecoenv.2004.06.010.
Pascual, M. B., Echevarria, V., Gonzalo, M. J., Hernandez-Apaolaza, L., 2016: Silicon addition to soybean (Glycine max L.) plants alleviate zinc deficiency. Plant Physiology and Biochemistry 108, 132–138, DOI: 10.1016/j.plaphy.2016.07.008. Epub 2016 Jul 11.
Pavlovic, J., Samardzic, J., Maksimovic, V., Timotijevic, G., Stevic, N., Laursen, K. H., Hansen, T. H., Husted, S., Schjoerring, J. K., Liang, Y., Nikolic, M., 2013: Silicon alleviates iron deficiency in cucumber by promoting mobilization of iron in the root apoplast. New Phytologist 198 (4), 1096-1107, DOI: 10.1111/nph.12213.
Rajasheker, G., Jawahar, G., Jalaja, N., Kumar, S. A., Kumari, P. H., Punita, D. L., Karumanchi, A. R., Reddy, P. S., Rathnagiri, P., Sreenivasulu, N., Kishor, P. B. K., 2019: Role and regulation of osmolytes and ABA interaction in salt and drought stress tolerance. Plant Signaling Molecules Woodhead Publishing. p. 417–436, DOI: 10.1016/B978-0-12-816451-8.00026-5.
Rehman, S., Abbas, G., Shahid, M., Saqib, M., Farooq, A. B. U., Hussain, M., Murtaza, B., Amjad, M., Naeem, M.A., Farooq, A., 2019: Effect of salinity on cadmium tolerance, ionic homeostasis and oxidative stress responses in Conocarpus exposed to cadmium stress: Implications for phytoremediation. Ecotoxicology and Environmental Safety 171, 146–153, DOI: 10.1016/j.ecoenv.2018.12.077.
Rizwan, M., Ali, S., Ibrahim, M., Farid, M., Adrees, M., Bharwana, S. A., Zia-ur-Rehman, M., Qayyum, M. F., Abbas, F., 2015: Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: a review. Environmental Science and Pollution Research 22, 15416–15431, DOI: 10.1007/s11356-015-5305-x.
Schaller, J., Puppe, D., Kaczorek, D., Ellerbrock, R., Sommer, M., 2021: Silicon cycling in soils revisited. Plants 10: 295, DOI: 10.3390/plants10020295.
Shabala, S., Cuin, T. A., 2008: Potassium transport and plant salt tolerance. Physiologia Plantarum 133, 651-669, DOI: 10.1111/j.1399-3054.2007.01008.x
Shi, Y., Zhang, Y., Yao, H., Wu, J., Sun, H., Gong, H., 2014: Silicon improves seed germination and alleviates oxidative stress of bud seedlings in tomato under water deficit stress. Plant Physiology and Biochemistry 78, 27–36, DOI: 10.1016/j.plaphy.2014.02.009.
Soylemezoglu, G., Demir, K., Inal, A., Gunes, A., 2009: Effect of silicon on antioxidant and stomatal response of two grapevine (Vitis vinifera L.) rootstocks grown in boron toxic, saline and boron toxic-saline soil. Scientia Horticulturae 123, 240–246, DOI: 10.1016/j.scienta.2009.09.005.
Stevens, R. M., Harvey, G., Partington, D. L., Coombe, B. G., 1999: Irrigation of grapevines with saline water at different growth stages: effects on soil, vegetative growth and yield. Australian Journal of Agricultural Research 50, 343-355, DOI: 10.1111/j.1755-0238.2011.00145.x.
Stevic, N., Korac, J., Pavlovic, J., Nikolic, M., 2016: Binding of transition metals to monosilicic acid in aqueous and xylem (Cucumis sativus L.) solutions: a low-T electron paramagnetic resonance study. BioMetals 29, 945–951, DOI:10.1007/s10534-016-9966-9.
Sulieman, S., Tran, L. S., 2015: Phosphorus homeostasis in legume nodules as an adaptive strategy to phosphorus deficiency. Plant Science 239, 36–43, DOI: 10.1016/j.plantsci.2015.06.018.
Thorne, S. J., Hartley, S. E., Maathuis, F. J. M., 2020: Is silicon a panacea for alleviating drought and salt stress in crops? Frontiers in Plant Science 11, 1221, DOI: 10.3389/fpls.2020.01221.
Torabi, F., Majd, A., Enteshari, S., 2015: The effect of silicon on alleviation of salt stress in borage (Borago officinalis L.). Journal of Soil Science and Plant Nutrition 61, 788–798, Doi: 10.1080/00380768.2015.1005540.
Updhyaya, A., Sankhla, D., T. D., Davis, T. D., Sankhla, N., Smidth, B. N., 1985: Effect of paclobutrazol on the activities of some enzymes of activated oxygen metabolism and lipid peroxidation in senescing soybean leaves. Journal of Plant Physiology 121,453-461, DOI: 10.1016/S0176-1617(85)80081-X.
Wang, X. S., Han, J. G., 2007: Effects of NaCl and silicon on ion distribution in the roots, shoots and leaves of two alfalfa cultivars with different salt tolerance. Journal of Soil Science and Plant Nutrition 53, 278–285, DOI: 10.1111/j.1747-0765.2007.00135.x.
Wang, S., Liu, P., Chen, D., Yin, L., Li, H., Deng, X., 2015: Silicon enhanced salt tolerance by improving the root water uptake and decreasing the ion toxicity in cucumber. Frontiers in Plant Science 6, 759, DOI:10.3389/fpls.2015.00759.
Xu, C. X., Ma, Y. P., Liu, Y. L., 2015: Effects of silicon (Si) on growth, quality and ionic homeostasis of aloe under salt stress. South African Journal of Botany 98, 26–36, DOI: 10.1016/j.sajb.2015.01.008.
Yan, G., Fan, X., Tan, L., Yin, C., Li, T., Liang, Y., 2021: Root silicon deposition and its resultant reduction of sodium bypass flow is modulated by OsLsi1 and OsLsi2 in rice. Plant Physiology and Biochemistry 158, 219–227, DOI: 10.1016/j.plaphy.2020.11.015.
Yang, Y., Guo, Y., 2018: Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytologist 217, 523–539, DOI: 10.1111/nph.14920.
Yassen, A., Abdallah, E., Gaballah, M., Zaghloul, S., 2017: Role of silicon dioxide nano fertilizer in mitigating salt stress on growth, yield and chemical composition of Cucumber (Cucumis sativus L.). International Journal of Agricultural Research, 22, 130–135, DOI: 10.3923/ijar.2017.130.135.
Yeo, A. R., Flowers, S. A., Rao, G., Welfare, K., Senanayake, N., Flowers, T. J., 1999: Silicon reduces sodium uptake in rice (Oryza sativa L.) in saline conditions and this is accounted for by a reduction in the transpirational bypass flow. Plant Cell Environment 22, 559–565, DOI: 10.1046/j.1365-3040.1999.00418.x.
Yin, L., Wang, S., Li, J., Tanaka, K., Oka, M., 2013: Application of silicon improves salt tolerance through ameliorating osmotic and ionic stresses in the seedling of Sorghum bicolor. Acta Physiologiae Plantarum 35, 3099–3107, DOI: 10.1007/s11738-013-1343-5.
Zargar, S. M., Mahajan, R., Bhat, J. A., Nazir, M., Deshmukh, R., 2019: Role of silicon in plant stress tolerance: Opportunities to achieve a sustainable cropping system. 3 Biotech 9 (3), 73, DOI: 10.1007/s13205-019-1613-z.
Zhang, Y., Liang, Y., Zhao, X., Jin, X., Hou, L., Shi, Y., Ahammed, G. J., 2019: Silicon compensates phosphorus deficit-induced growth inhibition by improving photosynthetic capacity, antioxidant potential, and nutrient homeostasis in tomato. Agronomy 9, 733, DOI: 10.3390/agronomy9110733.
Zhu, Y. X., Gong, H. J., 2014: Beneficial effects of silicon on salt and drought tolerance in plants. Agronomy for Sustainable Development 34, 455–472, DOI: 10.1007/s13593-013-0194-1.
Zhu, Y. X., Xu, X. B., Hu, Y. H., Han, W. H., Yin, J. L., Li, H. L., Gong, H. J., 2015: Silicon improves salt tolerance by increasing root water uptake in Cucumis sativus L. Plant Cell Reports 34, 1629–1646, DOI: 10.1007/s00299-015-1814-9.
Zhu, Y. X., Guo, J., Feng, R., Jia, J. H., Han, W. H., Gong, H. J., 2016: The regulatory role of silicon on carbohydrate metabolism in Cucumis sativus L. under salt stress. Plant and Soil 406, 231–249, DOI: 10.1007/s11104-016-2877-2.
Zhu, Y. X., Gong, H. J., Yin, J. L., 2019a: Role of silicon in mediating salt tolerance in plants: a review. Plants 8, 147, DOI: 10.3390/plants8060147.
Zhu, Y. X., Jia, J. H., Yang, L., Xia, Y. C., Zhang, H. L., Jia, J. B., Zhou, R., Nie, P. Y., Yin, J. L., Ma, D. F., Liu, L.C., 2019b: Identification of cucumber circular RNAs responsive to salt stress. BMC Plant Biology 19, 164, DOI: 10.1186/s12870-019-1712-3.